 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| ILP-HP2H3 | Human | PE-Labeled Human IL-1 RAcP / IL-1 R3 Protein, His Tag (Site-specific conjugation) | 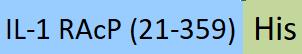 |
||
| ILP-HF223 | Human | FITC-Labeled Human IL-1 RAcP / IL-1 R3 Protein, His Tag | 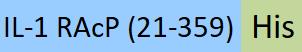 |

|
|
| ILP-H82F5 | Human | Biotinylated Human IL-1 RAcP / IL-1 R3 Protein, Fc,Avitag™ (MALS verified) | 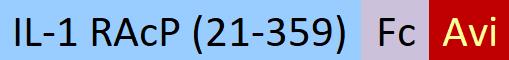 |
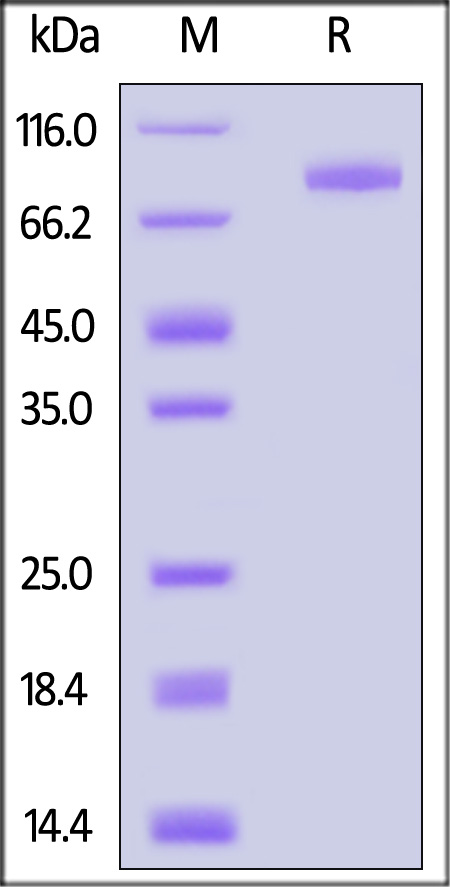
|
|
| ILP-C52H5 | Cynomolgus | Cynomolgus IL-1 RAcP / IL-1 R3 Protein, His Tag |  |
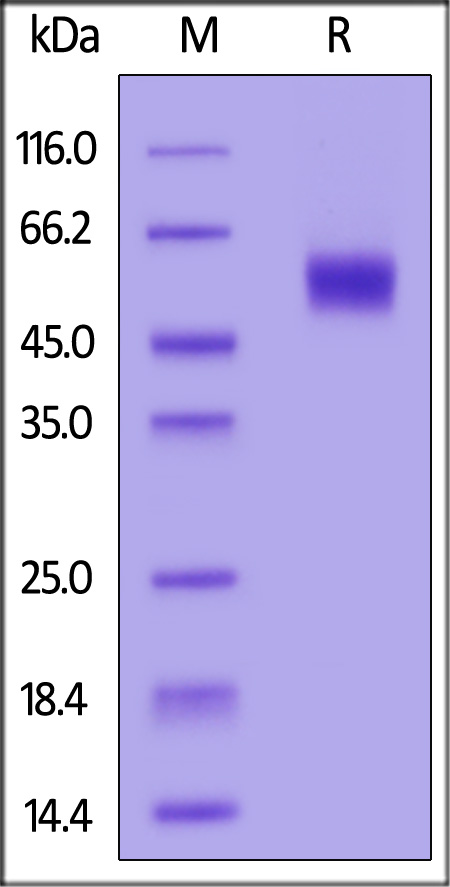
|
|
| ILP-M52H5 | Mouse | Mouse IL-1 RAcP / IL-1 R3 Protein, His Tag | 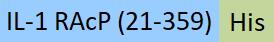 |

|
|
| ILP-H82E5 | Human | Biotinylated Human IL-1 RAcP / IL-1 R3 Protein, His,Avitag™ (MALS verified) |  |
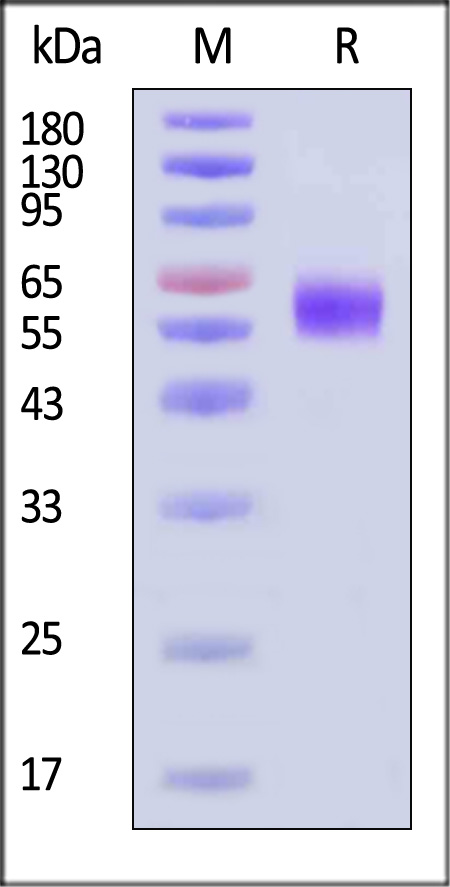
|
|
| ILP-H5256 | Human | Human IL-1 RAcP / IL-1 R3 Protein, Fc Tag |  |

|
|
| ILP-H5225 | Human | Human IL-1 RAcP / IL-1 R3 Protein, His Tag (MALS verified) |  |
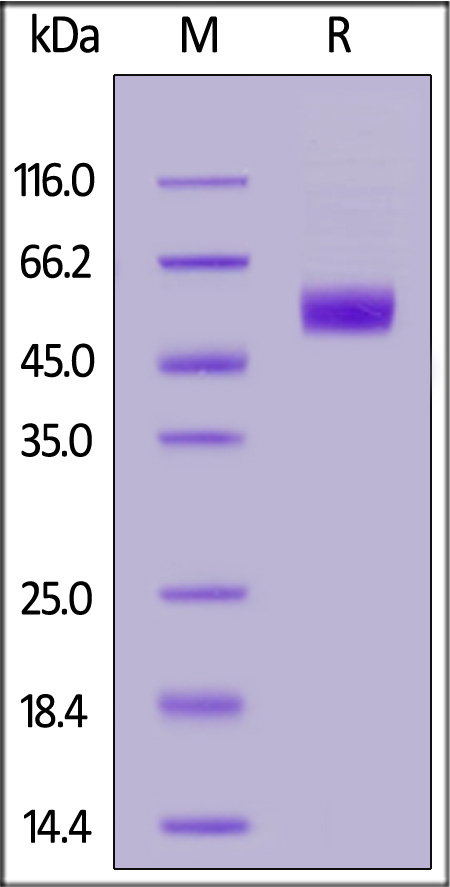
|
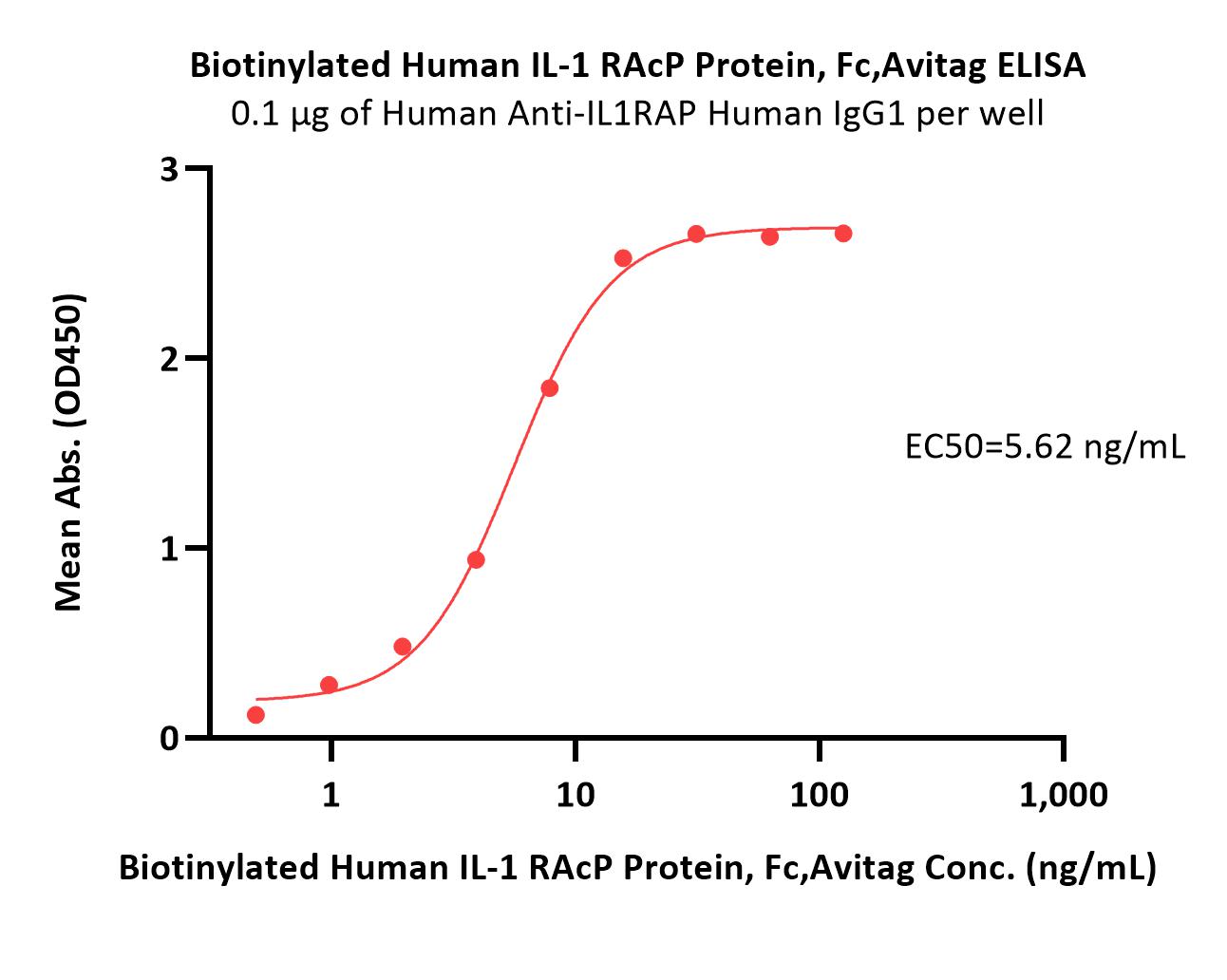
Immobilized Human Anti-IL1RAP Human IgG1 at 1 μg/mL (100 μL/well) can bind Biotinylated Human IL-1 RAcP Protein, Fc,Avitag (Cat. No. ILP-H82F5) with a linear range of 0.5-16 ng/mL (QC tested).
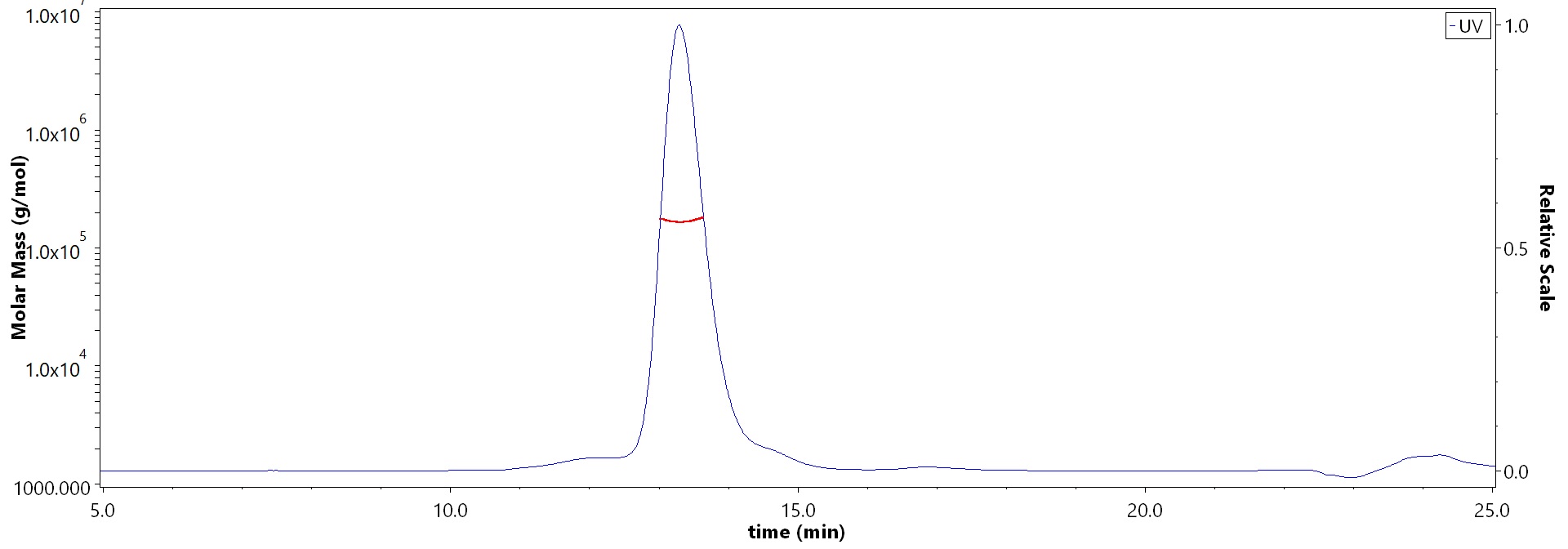
The purity of Biotinylated Human IL-1 RAcP Protein, Fc,Avitag (Cat. No. ILP-H82F5) is more than 85% and the molecular weight of this protein is around 155-175 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Nidanilimab | CAN-04 | Phase 2 Clinical | Lund University Foundation | Solid tumours; Biliary Tract Neoplasms; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| CCTx-001 | CCTx-001 | Phase 2 Clinical | Advesya SAS | Leukemia, Myeloid, Acute | Details |
| SAR-445399 | SAR-445399 | Phase 1 Clinical | Sanofi | Inflammation | Details |
| GSK3903371 | GSK3903371 | Clinical | Glaxosmithkline Plc | Neoplasms | Details |
| Nidanilimab | CAN-04 | Phase 2 Clinical | Lund University Foundation | Solid tumours; Biliary Tract Neoplasms; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| CCTx-001 | CCTx-001 | Phase 2 Clinical | Advesya SAS | Leukemia, Myeloid, Acute | Details |
| SAR-445399 | SAR-445399 | Phase 1 Clinical | Sanofi | Inflammation | Details |
| GSK3903371 | GSK3903371 | Clinical | Glaxosmithkline Plc | Neoplasms | Details |
This web search service is supported by Google Inc.
