Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Fibrillas preformadas (PFF)--Un enfoque novedoso para modelar la neurodegeneración
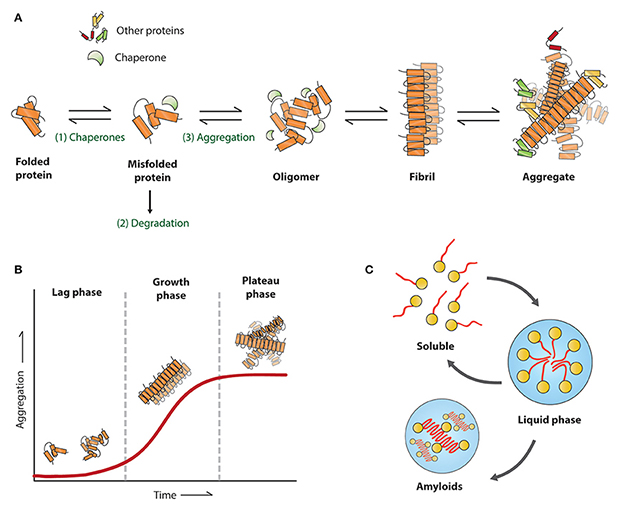
La agregación de proteínas es una de las principales características patológicas de las enfermedades neurodegenerativas, como la enfermedad de Alzheimer (EA), la enfermedad de Parkinson (EP), la esclerosis lateral amiotrófica (ELA) y la enfermedad de Huntington (EH). En condiciones patológicas, la Tau, la beta amiloide, la alfa-sinucleína, la TDP-43, la huntingtina y otras proteínas se integran en una amplia gama de estructuras indeseables, que van desde oligómeros y ensamblajes prefibrilares hasta agregados altamente ordenados. La estructura fibrilar representa una fase de crecimiento rápido de la agregación de proteínas, ya que estas fibrillas son "activas" y reclutan rápidamente monómeros para su elongación. Además, estas fibrillas se rompen aleatoriamente en fragmentos más cortos que podrían actuar como "semillas", que se transmiten a otras células y reclutan independientemente monómeros para formar nuevas fibrillas. Las fibrillas preformadas (PFF) son fibrillas activas formadas in vitro que tienen esta actividad de "siembra" y son capaces de reclutar continuamente proteínas patológicas endógenas solubles para formar agregados y finalmente inducir patologías neurodegenerativas.
El establecimiento de modelos de enfermedad fiables es crucial para descubrir los mecanismos patológicos, evaluar la eficacia de las intervenciones terapéuticas y valorar la seguridad de los candidatos a fármacos. En comparación con los enfoques tradicionales de modelización de enfermedades, la patología inducida por PFF no depende de la edición de genes, ni de daños químicos o físicos, y puede imitar mejor los procesos de estados patológicos que se producen de forma natural. Por lo tanto, los PFF son un enfoque novedoso para modelar las enfermedades neurodegenerativas.
PFFs could be generated from monomers either by incubating at 37°C and shaking, or by heparin induction. Quality control and precise initial preparation are important for successful experiments. One important aspect of successful induction of PFFs is the use of high-quality monomer with high purity, high concentration and correct conformation. Meanwhile, since the application of PFFs is cellular and animal experiments, the control of endotoxin is also an important factor. In addition, prior to the use of PFFs, PFFs should become a length of 50 nm or shorter via ultrasound to ensure the recruiting activity of PFFs as well as to facilitate the endocytosis of PFFs.
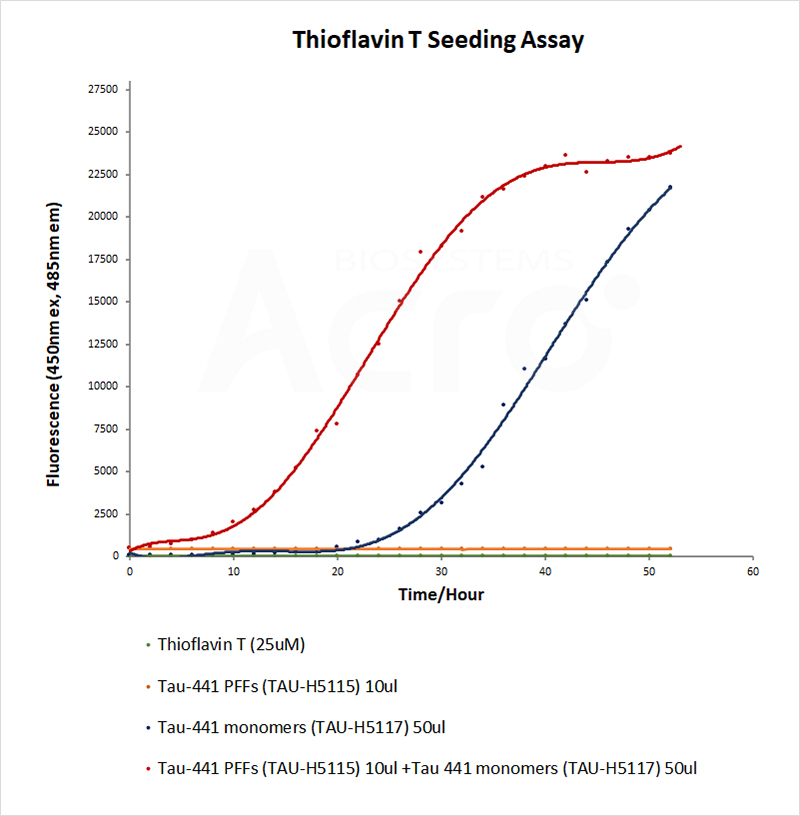
The morphology and activity of PFFs could be verified by electron microscope and thioflavin T (ThT) fluorescence assay respectively. Successfully induced PFFs show fibril structure under electron microscope. ThT assay is a classic assay to detect β-sheet structures. PFFs acquire more and more β-sheet structures as they recruit monomers, and when ThT binds to the β-sheet structure, the fluorescence value increases, thus reflecting the activity of PFFs.
Aneuro es la marca de ACROBiosystems que se centra en el campo de la neurociencia. Aneuro proporciona PFF de Tau-441, PFF de alfa-sinucleína, PFF de beta amiloide, PFF de TDP-43 y PFF de SOD-1, apoyando y acelerando el establecimiento de modelos neurodegenerativos fiables.
| Molecule | Cat. No. | Product Description | Monomer Source | Expression System | Preorder/Order |
|---|---|---|---|---|---|
| Tau | TAU-H5115 | Human Tau-441/2N4R Pre-formed Fibrils Protein, Tag Free | TAU-H5117 | E.coli | |
| TAU-H5116 | Human Tau-441 K18 Pre-formed Fibrils Protein, Tag Free | NA | E.coli | ||
| TAU-H5113 | Human Tau-441 K18 (P301L) Pre-formed Fibrils Protein, Tag Free | TAU-H5118 | E.coli | ||
| Alpha-Synuclein | ALN-H5114 | Human Alpha-Synuclein (A53T) Pre-formed Fibrils Protein, Tag Free | ALN-H5116 | E.coli | |
| ALN-H5115 | Human Alpha-Synuclein Pre-formed Fibrils Protein, Tag Free | ALN-H5214 | E.coli |
Meanwhile, alpha-synuclein PFFs,Amyloid beta PFFs,TDP-43 PFFs,SOD-1 PFFs are under development. Deje un mensaje
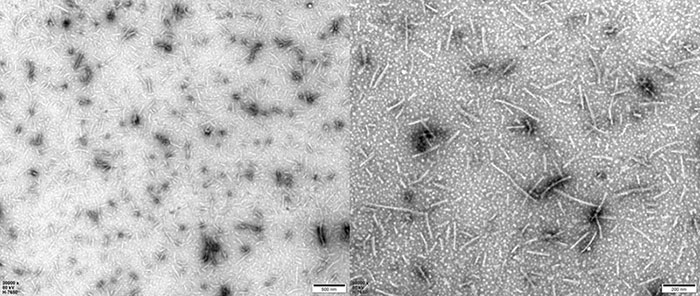
TEM of Human Tau-441/2N4R Pre-formed Fibrils Protein (Cat. No. TAU-H5115).
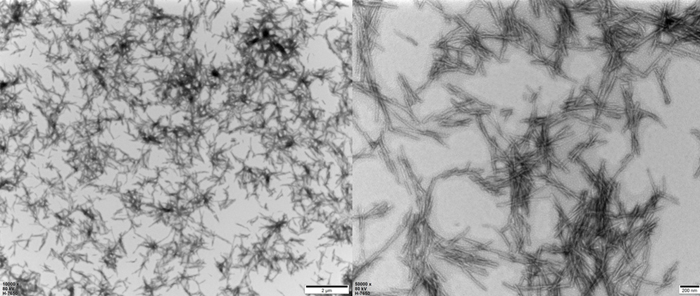
TEM of Human Tau-441 K18 (P301L) Pre-formed Fibrils Protein (Cat. No. TAU-H5113).
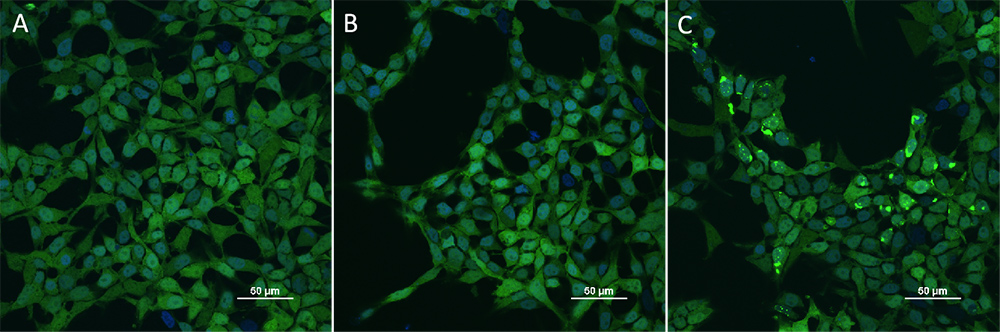
HEK293/Human Tau(GFP) Stable Cell Line were transduced with Human Tau-441 / 2N4R Pre-formed Fibrils Protein, Tag Free (ThT active) (Cat. No. TAU-H5115) and Human Tau-441 / 2N4R Protein, Tag Free (MALS verified) (Cat. No. TAU-H5117) respectively. The fluorescence of GFP-Tau (Green) and DAPI (Blue) were detected by confocal microscope. A. Lipo2000 transduction. B. Lipo2000 and Human Tau-441 / 2N4R Protein, Tag Free (MALS verified) transduction. C. Lipo2000 and Human Tau-441 / 2N4R Pre-formed Fibrils Protein, Tag Free (ThT active) transduction. Scale bars, 50 μm.
1. Stroo E, Koopman M, Nollen EA, Mata-Cabana A. Cellular Regulation of Amyloid Formation in Aging and Disease. Front Neurosci (2017). doi: 10.3389/fnins.2017.00064. PMID: 28261044; PMCID: PMC5306383.
2. Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem (2011). doi: 10.1074/jbc.M110.209296. Epub 2011 Mar 3. PMID: 21372138; PMCID: PMC3083182.
This web search service is supported by Google Inc.