 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Citoquinas de grado GMP
![]() Fabricado y sometido a pruebas de control de calidad bajo el cumplimiento de las GMP.
Fabricado y sometido a pruebas de control de calidad bajo el cumplimiento de las GMP.
![]() Diseñado bajo las normas ISO 9001:2015 e ISO 13485:2016
Diseñado bajo las normas ISO 9001:2015 e ISO 13485:2016
![]() Materiales libres de animales
Materiales libres de animales
![]() Materiales adquiridos de proveedores aprobados
Materiales adquiridos de proveedores aprobados
![]() Salas limpias ISO 5 y equipos de llenado automático
Salas limpias ISO 5 y equipos de llenado automático
![]() Personal cualificado y bien formado
Personal cualificado y bien formado
![]() Documentos relacionados con la calidad revisados y aprobados por el control de calidad
Documentos relacionados con la calidad revisados y aprobados por el control de calidad
![]() Registros de producción y control de lotes completos
Registros de producción y control de lotes completos
![]() Mantenimiento y calibración de los equipos
Mantenimiento y calibración de los equipos
![]() Validación de los procedimientos analíticos
Validación de los procedimientos analíticos
![]() Estudios de estabilidad realizados
Estudios de estabilidad realizados
![]() Archivos completos de apoyo normativo
Archivos completos de apoyo normativo


![]() SDS-PAGE>95%
SDS-PAGE>95%
![]() Nivel de endotoxinas inferior a 10 EU/mg
Nivel de endotoxinas inferior a 10 EU/mg
![]() Contenido residual de ADN de la célula huésped inferior a 0,02ng/μg
Contenido residual de ADN de la célula huésped inferior a 0,02ng/μg
![]() Contenido residual de proteínas de la célula huésped inferior a 0,5ng/ug
Contenido residual de proteínas de la célula huésped inferior a 0,5ng/ug
![]() Actividad biológica >0,8 x 107 UI/mg (Referencia de la IL-15 humana de la OMS (código NIBSC: 90/530) de serie)
Actividad biológica >0,8 x 107 UI/mg (Referencia de la IL-15 humana de la OMS (código NIBSC: 90/530) de serie)
![]() Pruebas microbianas
Pruebas microbianas
![]() Pruebas de micoplasma
Pruebas de micoplasma
![]() Prueba de virus in vitro
Prueba de virus in vitro
![]() Consistencia entre lotes
Consistencia entre lotes
![]() Amplio soporte de datos de estabilidad (aceleración, congelación-descongelación, largo plazo, verificación de la estabilidad en el transporte)
Amplio soporte de datos de estabilidad (aceleración, congelación-descongelación, largo plazo, verificación de la estabilidad en el transporte)
![]() Normas estrictas de control de calidad:
Normas estrictas de control de calidad:
- 16 normas de control de calidad.
- Excelente perfil de seguridad (pruebas de esterilidad, micoplasma, endotoxina e impurezas residuales).
- Alta estabilidad y consistencia entre lotes.
![]() Sistema de gestión de calidad de grado GMP
Sistema de gestión de calidad de grado GMP
- ISO 5 salas limpias utilizadas para el llenado.
- Las materias primas y los materiales de embalaje están registrados.
- Se dispone de instalaciones para realizar auditorías en línea e in situ.
![]() Acelerar la aprobación reglamentaria mundial de los productos biológicos
Acelerar la aprobación reglamentaria mundial de los productos biológicos
- Se dispone de un conjunto completo de documentos reglamentarios.
- Los informes de validación de los métodos de análisis están disponibles previa solicitud.
- FDA DMF filed.
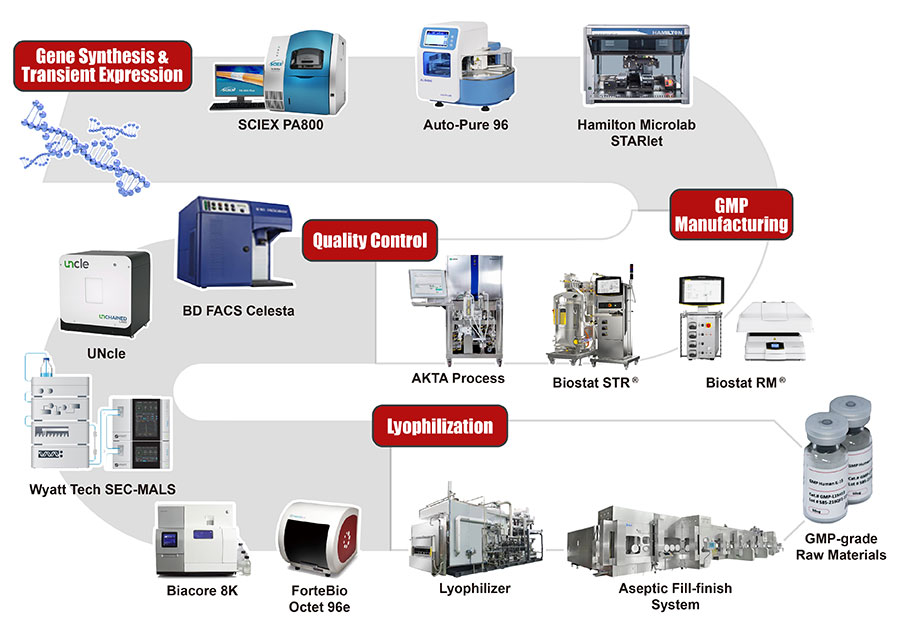
En ACROBiosystems, hemos desarrollado más de 5.000 productos de proteínas recombinantes y acumulado más de 10 años de experiencia en el desarrollo y la fabricación de proteínas. Ofrecemos un servicio integral que abarca desde la síntesis de genes y la construcción de vectores hasta la expresión y purificación de proteínas. Esto incluye todos los pasos, desde el diseño de proteínas, la optimización de codones, la síntesis de genes, la purificación y la ampliación. Se pueden seleccionar diferentes sistemas de expresión, métodos de purificación y etiquetas/etiquetados proteínicos en función de sus necesidades para maximizar el éxito de su terapia.
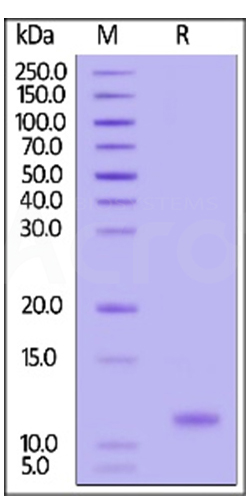
High purity than 90% of GMP Human IL-15
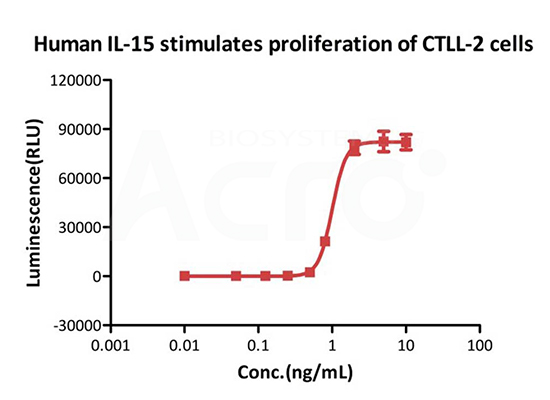
GMP Human IL-15 (Cat. No. GMP-L15H13) stimulates the proliferation of CTLL-2 cells. The EC50 for this effect is 1.004 ng/mL, corresponding to a specific activity of > 0.8ⅹ10^7 IU/mg, which is calibrated against human IL- 15 WHO International Standard (NIBSC code: 95/554).
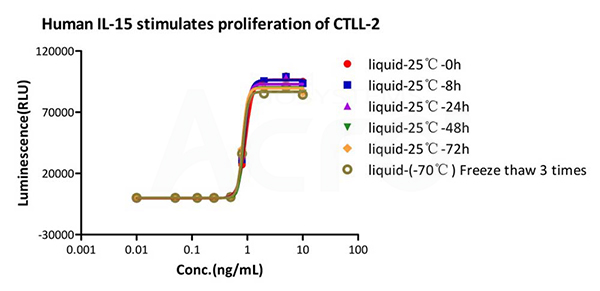
GMP Human IL-15 (GMP-L15H13) is stable in undiluted samples at 25℃ for 72 hours and freeze-thaw 3 times without performance reduction.
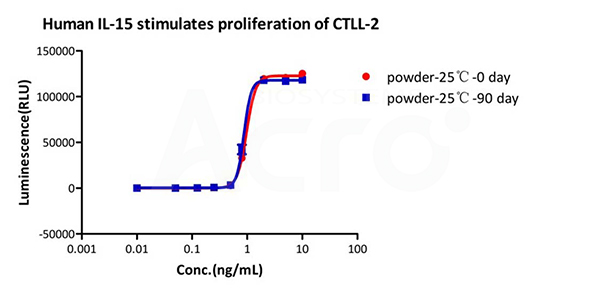
GMP Human IL-15 (GMP-L15H13) is stable in undiluted samples at 25 ℃ for 90 days without performance reduction performance reduction.
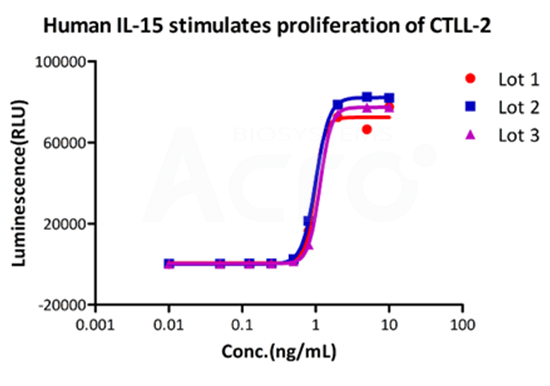
Bioactivity of three different lots of GMP Human IL-15 (GMP-L15H13) verified by cell-based assay, and the result shows very high batch-to-batch consistency.
This web search service is supported by Google Inc.
