
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Kits de ensayo ELISA para el análisis cuantitativo de anticuerpos terapéuticos en muestras de suero

La farmacocinética (PK) es una rama de la ciencia dedicada al análisis cuantitativo de la absorción, la distribución, el metabolismo y la excreción de las moléculas de los fármacos dentro del cuerpo de un organismo vivo. Todos los estudios preclínicos y clínicos incluyen la medición de la concentración sérica del fármaco, tanto en animales como en pacientes, en diferentes momentos tras la administración del fármaco. El resultado es un importante indicador de las propiedades farmacocinéticas del fármaco, y es pertinente para las recomendaciones de dosificación.
El auge del mercado de los fármacos biológicos, impulsado por la avalancha de éxitos de los anticuerpos monoclonales, trae consigo la necesidad de realizar ensayos estándar de alto rendimiento para evaluar el contenido de los mAbs en las muestras de suero. Tradicionalmente, los análisis se llevan a cabo mediante ELISA indirecto, o con la ayuda de anticuerpos antidroga (AA), cada uno con sus propios pros y contras. Hemos desarrollado una serie de ensayos basados en el método ELISA competitivo. El ensayo emplea un mAb biotinilado contra el mismo objetivo como trazador para competir con el analito no marcado, y utiliza la interacción entre la biotina y la estreptavidina para la eventual lectura. Este método no depende de los AAs, y también alivia el problema de fondo que a menudo se produce con el formato de ensayo tradicional.
Hemos lanzado kits para los estudios de CTLA-4, PD-1 y HER-2, respectivamente, tanto en humanos como en animales de experimentación comunes.
| Cat. No. | Descripción del producto | Tamaño |
|---|---|---|
| EPH-V1 | ELISA Assay Kit for Anti-PD-1 h-mAb in Human Serum | 96/480 tests |
| EHM-V1 | ELISA Assay Kit for Anti-HER-2 h-mAb in Mouse Serum | 480 tests |
The serum samples contain many factors that may potentially interfere with the indirect ELISA result. This is the major reason for the background issue (Fig. 1A). Therefore, a series of testing need to be performed to determine the minimum required dilution (MRD) before an experiment can be conducted. This can be time consuming and the results can vary. On the other hand, the competitive ELISA method we employed for our kit does not have a background issue. As shown in Fig 1B, dilutions up to 1:5 does not produce any background at all.
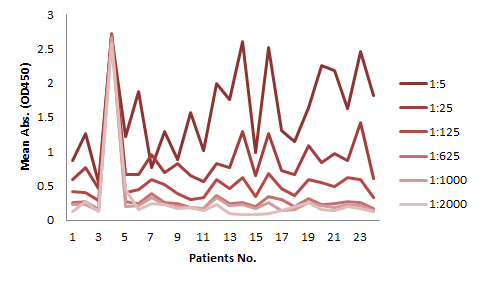
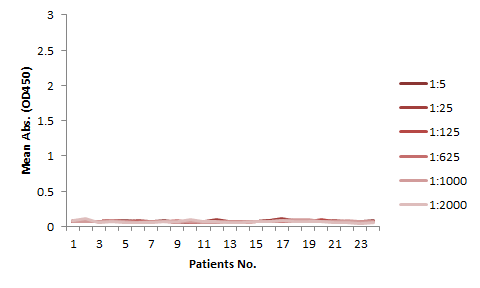
The assay kits can be used for studies of any mAbs share similar binding domain as our tracer biotinylated antibody.
For example, with our Anti-PD-1 mAb kit for human serum samples(Cat. No. EPH-V1), we have successfully measured five different anti-PD1 mAbs that are either on the market already or being tested in clinical trials.
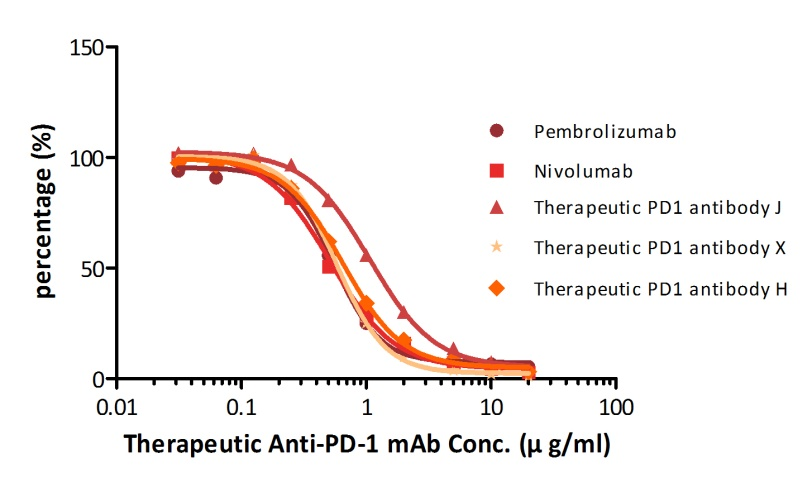
| Name | Pembrolizumab | Nivol*mab | Therapeutic PD-1 Antibody J, Human IgG4 | Therapeutic PD-1 Antibody X, Human IgG4 | Therapeutic PD-1 Antibody H, Human IgG4 |
|---|---|---|---|---|---|
| Detection Range (μg/ml) | 0.03125-20 | 0.03125-20 | 0.03125-20 | 0.03125-20 | 0.03125-20 |
| Sensitivity (μg/ml) | 0.15625 | 0.15625 | 0.15625 | 0.15625 | 0.15625 |
| %Recovery | 88-113 | 92-114 | 86-114 | 96-112 | 87-106 |
We install rigorous quality control program to ensure the lot-to-lot consistency of our products. Every batch of products are analyzed against our internal standards using various analytical methods. The product will be released only if all standards are met.
All kits components are analyzed for their stability using accelerated testing method. Based on the results shown in Fig. 3, the products can be stored at -80 for 4-6 months. The assay components are also tested after one or two freeze-thaw cycles. No significant activity loss was observed under either conditions.
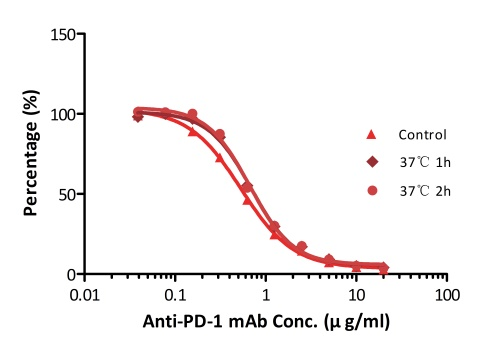
Fig. 3 ELISA using Anti-PD-1 h-mAb kit Human Serum (Cat. No. EPH-V1). The samples were incubated at 37℃ for 2 hours after reconstitution. No significant loss of activity was observed.
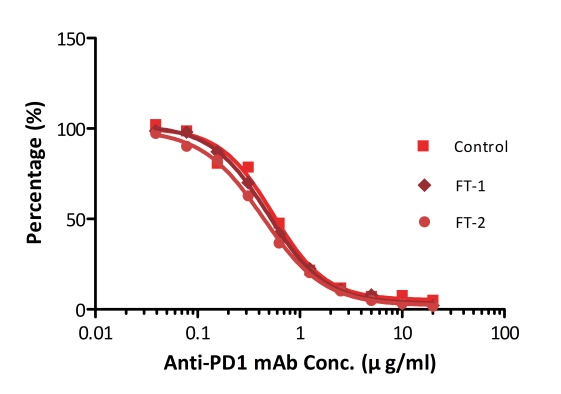
Fig. 4 ELISA using Anti-PD-1 h-mAb kit Human Serum (Cat. No. EPH-V1). The samples were subjected to zero, one, and two rounds of freeze-thawing cycles, respectively. No significant loss of activity was observed.
This web search service is supported by Google Inc.








