 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily. The TGF-β signaling pathway is involved in many cellular processes in both the adult organism and the developing embryo including cell growth, cell differentiation, apoptosis, cellular homeostasis, and other cellular functions.
TGF-β signaling in cancer is complex. On one hand, TGF-β can suppress tumor formation. On the other hand, it can also promote it. In normal cells and early-stage cancers, TGF-β signaling suppresses tumor formation by inhibiting cell growth and apoptosis. However, in advanced cancers, TGF-β signaling acquires a range of tumor-promoting effects through certain adaptation or signaling pathways bypass.
There are two mechanisms to explain the tumor-promoting effects of TGF-β:
1. The tumor uses TGF-β to promote the epithelial mesenchymal transition (EMT), thereby enhance the migration, invasion, infiltration, and extravasation of the tumor cells.
2. Through paracrine signaling, TGF-β can help forming the tumor microenvironment (TME), activate cancer-associated fibroblasts (CAF), promote angiogenesis, produce extracellular matrix (ECM), inhibit anti-tumor immune response, and eventually promote tumor metastasis (Figure 1).
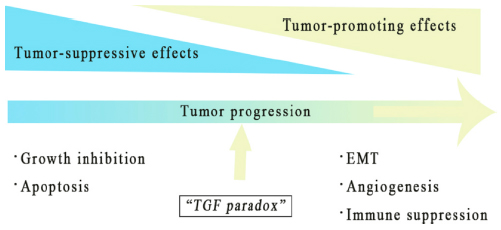
Figure 1 Tumor-promoting and suppressing effect of TGF-β
The TME is the environment around a tumor, including the surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and ECM. TEM is critical for tumor immune escape. It forms a barrier to protect the tumor from immune systems and finally leads to tumor metastasis. Therefore, TME is critical for tumor development and is one of the major issues in tumor clinical treatment that needs to be addressed.
The TME is induced by TGF-β through the following steps:
1. TGF-β activates/differentiates fibroblasts, mesenchymal stem cells, epithelial tumor cells, adipose tissue-derived stem cells, and endothelial cells (Figure 2A). The fibroblasts are key components of the TME. They promote immune escape and angiogenesis by supplying ECM and cytokines. Endothelium cells are located on the surface of blood and lymphatic vessels. Blood vessels nourish tumors by delivering blood/oxygen/nutrients, removing waste, and controlling the entry and exit of immune cells and other substances. TGF-β acts directly and indirectly on endothelial cells to promote angiogenesis and migration in TME (Figure 2b).
2. TGF-β suppresses the immune system by regulating the function of the immune cell populations in the tumor microenvironment (TME), which is dotted with a variety of innate and adaptive immune cells.
· TGF-β suppresses tumor adaptive immunity mainly by inhibiting T cell activation, proliferation, differentiation, and migration (Fig. 2C). TGF-β usually inhibits the differentiation of primary CD4 + T cells into different effector subtypes, but it can induce the transformation of primary T cells into regulatory T cells, which play an immunosuppressive role.
· TGF-β inhibits the activation and maturation of cytotoxic CD8 + T cells by inhibiting the processing of tumor antigens and the expression of DCs. TGF-β also inhibits the proliferation of CD8 + T cells by inhibiting the expression of IFNγ and IL2.
· Additionally, TGF-β promotes the expression of antigen-induced programmed cell death protein 1 (PD-1) in CD8 + T cells, leading to T cell failure.
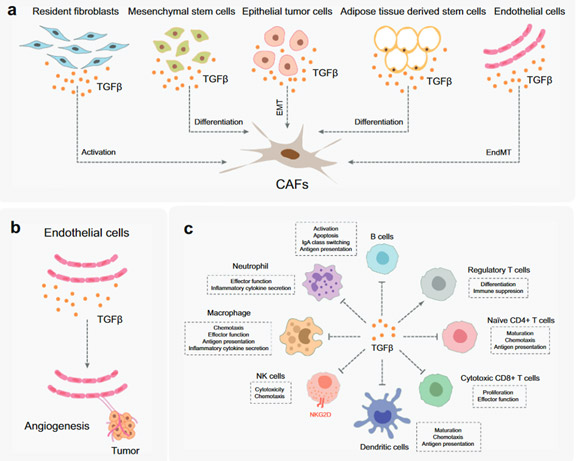 Figure 2. The function of TGF-β in TME
Figure 2. The function of TGF-β in TME
Since TGF-β is involved in many aspects of tumor genesis and development, the inhibition of TGF-β signal transduction provides multiple research targets for anti-tumor therapy, which has become a hot research field of new anti-cancer therapies.
Cancer cells often escape from TGF-β-induced cell suppression responses. That is why in most cases, cancer patients show recurrence symptoms after chemotherapy, targeted therapy, or radiation. The response of a single treatment regimen is limited. However, the combination therapy has become popular. We use an anti- TGF-β drug in combination with one of following treatments including immune checkpoint inhibitors (such as PD-1/PD-L1 antibodies), cytotoxic drugs, radiation therapy and cancer vaccines. According to the clinical data, the anti-tumor effect of the combination of PD-L1 antibody and TGF-β antibody is more effective than that of single-drug treatment (Table 1). However, the anticancer mechanism of TGF-β inhibitors is still unclear. The further understanding of the interaction between human body and TGF-β signaling is the key to improve clinical efficacy in the future.
Table 1 The combination of PD-L1 and TNF- β antibodies in clinical trials
| Drug name | The highest R&D status worldwide | Active company | Active Indications | target | Mechanism |
|---|---|---|---|---|---|
PM-8001 | phase II clinical trials | Pumis Biotechnology(Zhuhai)Co., Ltd. | solid tumor | PD-L1 | Programmed death-ligand 1 inhibitors(PD-L 1 Inhibitor); |
HBM-7015 | Preclinical development | Harbour BioMed | solid tumor | TGF-β1 | Transforming growth factor beta 1 inhibitors(TGF-β 1 Inhibitor); |
TGF-β signal transduction has attracted great attention in scientific and clinical studies due to its important role in tumor immune escape. An in-depth understanding of the mechanism of TGF-β can help to optimize the tumor treatment plans in clinical practice and provide a direction of new cancer drug development in the industry.
ACROBiosystems independently developed a series of TGF-beta 1, Latent TGF-beta 1, TGF-beta 3, TGF-beta RII proteins, and their biological activity has been verified by various technologies such as Cell based assay and ELISA with good feedback from our customers. Furthermore, the corresponding protocol is provided for free in order to help you shorten the R&D cycle.
product list
| Molecule | Cat. No. | Product Description |
|---|---|---|
| TGF-beta 1 | TG1-H4212 | ActiveMax® Human TGF-Beta 1 / TGFB1 Protein, Tag Free |
| TG1-H8217 | Biotinylated Human TGF-Beta 1 / TGFB1 Protein, Avitag™ | |
| TG1-M5218 | Mouse TGF-Beta 1 / TGFB1 Protein | |
| TGF-beta RII | TG2-H5252 | Human TGF-beta RII / TGFBR2 Protein, Fc Tag |
| TG2-H82E4 | Biotinylated Human TGF-beta RII / TGFBR2 Protein, His,Avitag™ | |
| TG2-H82F6 | Biotinylated Human TGF-beta RII / TGFBR2 Protein, Fc,Avitag™ (MALS verified) | |
| TG2-H52H5 | Human TGF-beta RII / TGFBR2 Protein, His Tag (MALS verified) | |
| TG2-M82H5 | Biotinylated Mouse TGF-beta RII / TGFBR2 Protein, His,Avitag™ | |
| LAP (TGF-beta 1) | LAP-H5245 | Human LAP (TGF-beta 1) Protein, His Tag |
| LAP-H82Q6 | Biotinylated Human LAP (TGF-beta 1) Protein, His,Avitag™ | |
| Latent TGF-beta 1 | TG1-H524x | Human Latent TGF-beta 1 / TGFB1 Protein, His Tag |
| TG1-H82Qb | Biotinylated Human LAP (TGF-beta 1) Protein, His,Avitag™ | |
| TG1-C5243 | Cynomolgus Latent TGF-beta 1 (C33S) Protein, His Tag |
Assay Data
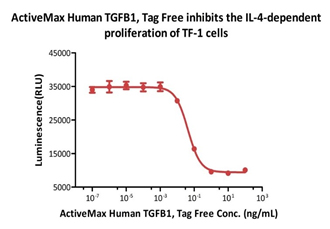
ActiveMax® Human TGFB1, Tag Free (Cat. No. TG1-H4212) inhibits the IL-4-dependent proliferation of TF-1 cells. The ED50 for this effect is 0.43-0.91 ng/mL (Routinely tested).
This web search service is supported by Google Inc.
