 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| GDF15 mAb - 01 | PCC | Metabolism | Solid tumor,Cancer cachexia |
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| CEA-C077 | Human | ClinMax™ Human GDF-15 ELISA Kit | |||
| GD5-H81Q3 | Human | Biotinylated Human GDF-15 / MIC-1 Protein, His,Avitag™ |  |

|
|
| GD5-M5149 | Mouse | Mouse GDF-15 / MIC-1 Protein, His Tag |  |
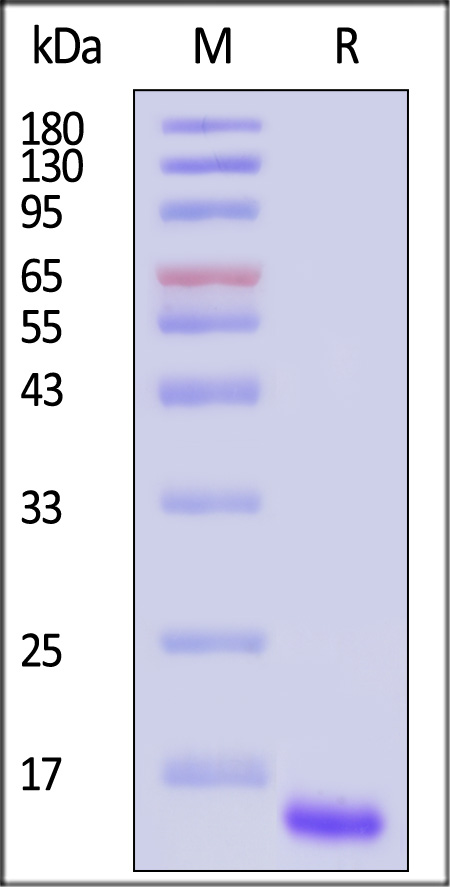
|
|
| GD5-H82F9 | Human | Biotinylated Human GDF-15 / MIC-1 Protein, Avitag™, Fc Tag (MALS verified) |  |
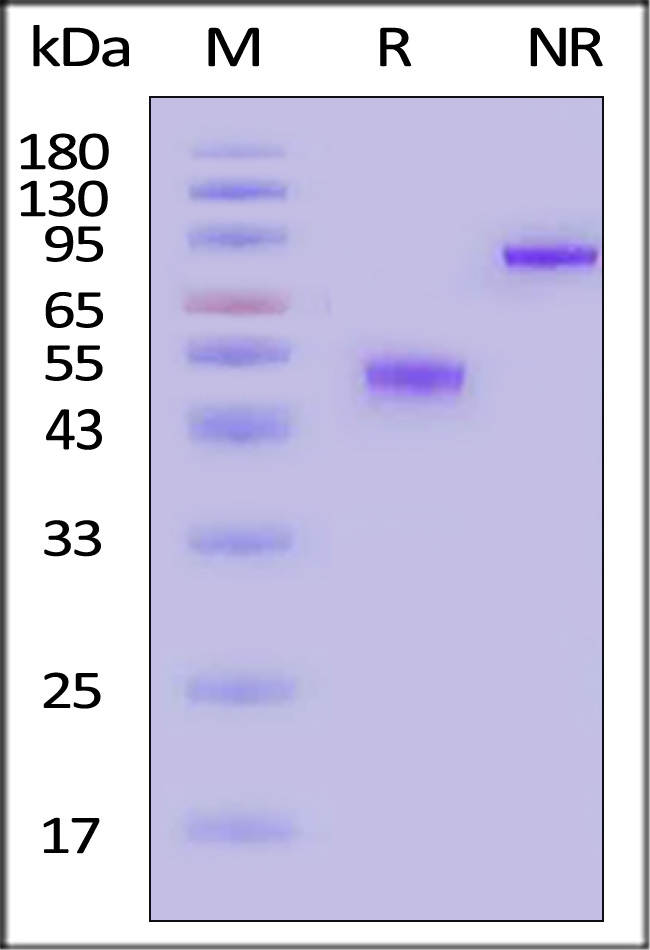
|
|
| GD5-C5148 | Cynomolgus | Cynomolgus GDF-15 / MIC-1 Protein, His Tag |  |

|
|
| GD5-H5269 | Human | Human GDF-15 / MIC-1 Protein, Fc Tag (MALS verified) |  |
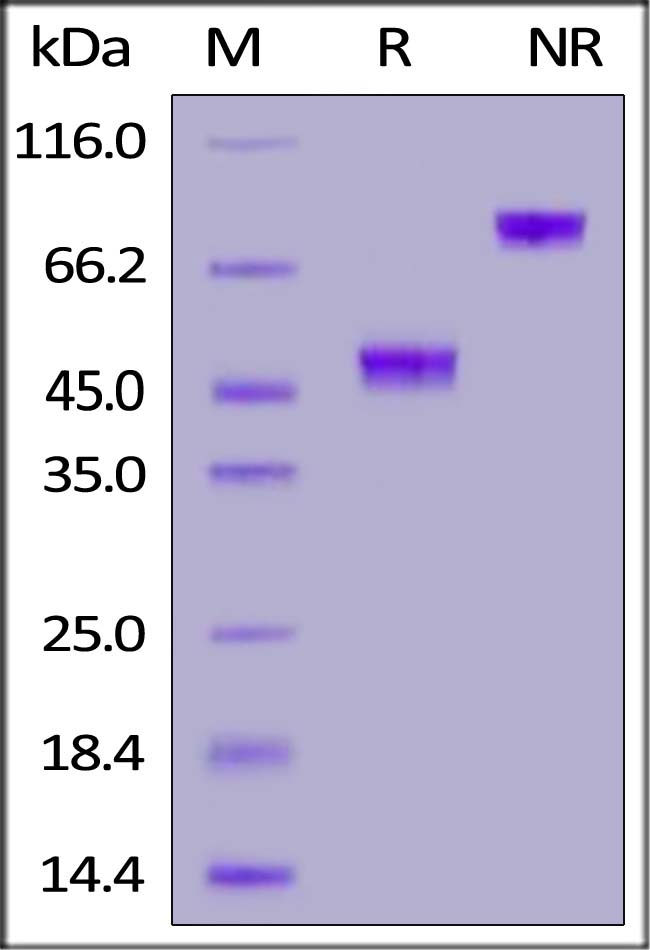
|
|
| GD5-H5149 | Human | Human GDF-15 / MIC-1 Protein, His Tag |  |
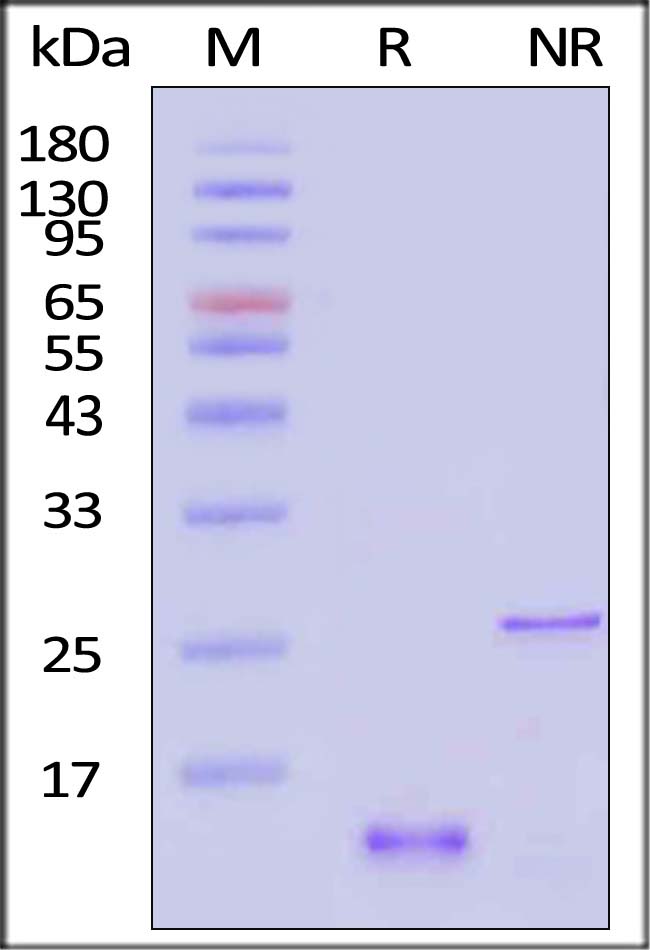
|
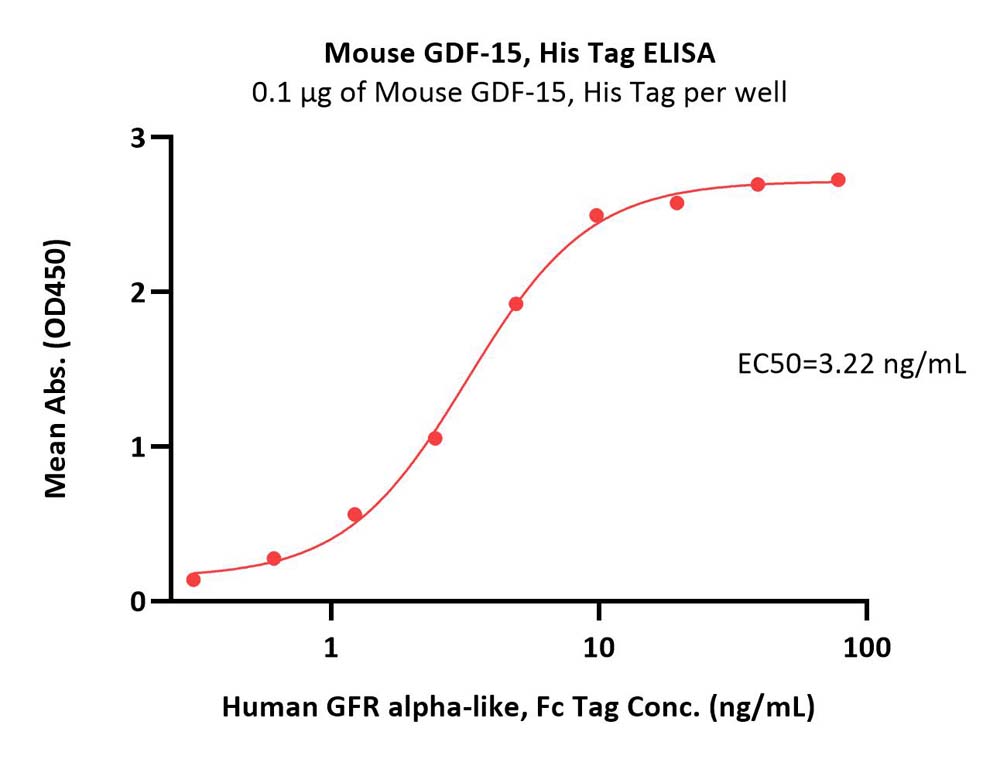
Immobilized Mouse GDF-15, His Tag (Cat. No. GD5-M5149) at 1 μg/mL (100 μL/well) can bind Human GFR alpha-like, Fc Tag (Cat. No. GFE-H5259) with a linear range of 0.3-10 ng/mL (QC tested).

The purity of Biotinylated Human GDF-15, Avitag, Fc Tag (Cat. No. GD5-H82F9) is more than 85% and the molecular weight of this protein is around 80-95 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| NGM-120 | NGM-120 | Phase 2 Clinical | Ngm Biopharmaceuticals Inc | Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Anorexia; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Cachexia; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Visugromab | CTL-002 | Phase 2 Clinical | CatalYm GmbH | Solid tumours; Urinary Bladder Neoplasms | Details |
| Ponsegromab | PF-06946860 | Phase 2 Clinical | Pfizer Inc | Ovarian Neoplasms; Heart Failure; Fatigue; Pancreatic Neoplasms; Anorexia; Feeding and Eating Disorders; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Cachexia; Carcinoma, Non-Small-Cell Lung | Details |
| GDF15 agonist (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| NN-9215 | Human-GDF15; MIC-1; NN-9215; LA-GDF15 | Phase 1 Clinical | Novo Nordisk A/S | Obesity | Details |
| CIN-109 | CIN-109; JNJ-9090 | Phase 1 Clinical | Janssen Sciences Ireland Unlimited Company | Obesity | Details |
| AV-380 | AV-380 | Phase 1 Clinical | Aveo Pharmaceuticals Inc | Cachexia | Details |
| NGM-120 | NGM-120 | Phase 2 Clinical | Ngm Biopharmaceuticals Inc | Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Anorexia; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Cachexia; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Visugromab | CTL-002 | Phase 2 Clinical | CatalYm GmbH | Solid tumours; Urinary Bladder Neoplasms | Details |
| Ponsegromab | PF-06946860 | Phase 2 Clinical | Pfizer Inc | Ovarian Neoplasms; Heart Failure; Fatigue; Pancreatic Neoplasms; Anorexia; Feeding and Eating Disorders; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Cachexia; Carcinoma, Non-Small-Cell Lung | Details |
| GDF15 agonist (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| NN-9215 | Human-GDF15; MIC-1; NN-9215; LA-GDF15 | Phase 1 Clinical | Novo Nordisk A/S | Obesity | Details |
| CIN-109 | CIN-109; JNJ-9090 | Phase 1 Clinical | Janssen Sciences Ireland Unlimited Company | Obesity | Details |
| AV-380 | AV-380 | Phase 1 Clinical | Aveo Pharmaceuticals Inc | Cachexia | Details |
This web search service is supported by Google Inc.
