 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| TR2-C52H7 | Cynomolgus | Cynomolgus TRAIL R2 / DR5 / TNFRSF10B Protein, His Tag (MALS verified) |  |
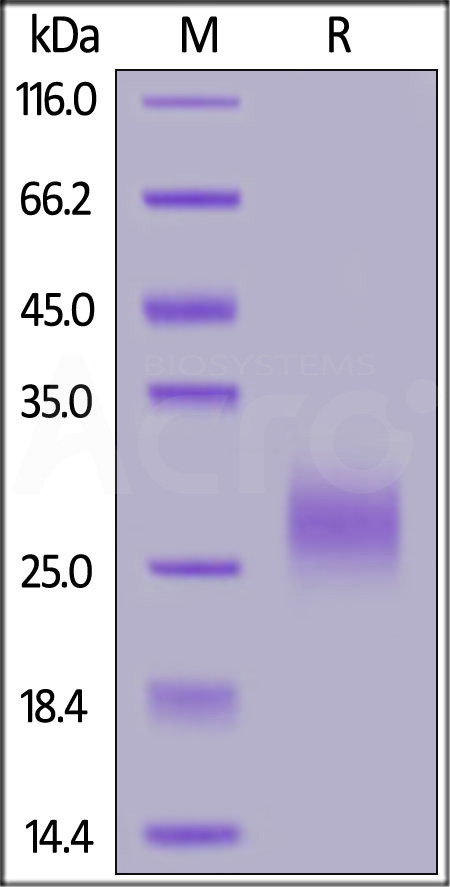
|
|
| TR2-M52H5 | Mouse | Mouse TRAIL R2 / DR5 / TNFRSF10B Protein, His Tag |  |
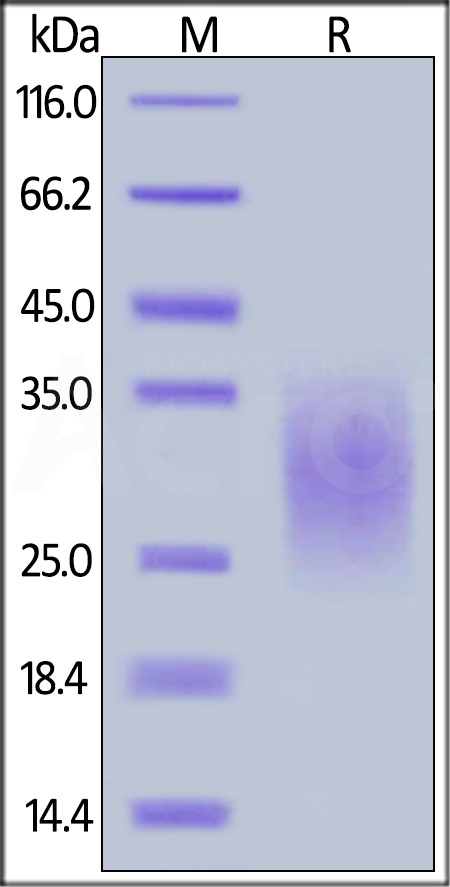
|
|
| TR2-H82E6 | Human | Biotinylated Human TRAIL R2 / DR5 / TNFRSF10B Protein, Avitag™,His Tag (MALS verified) |  |
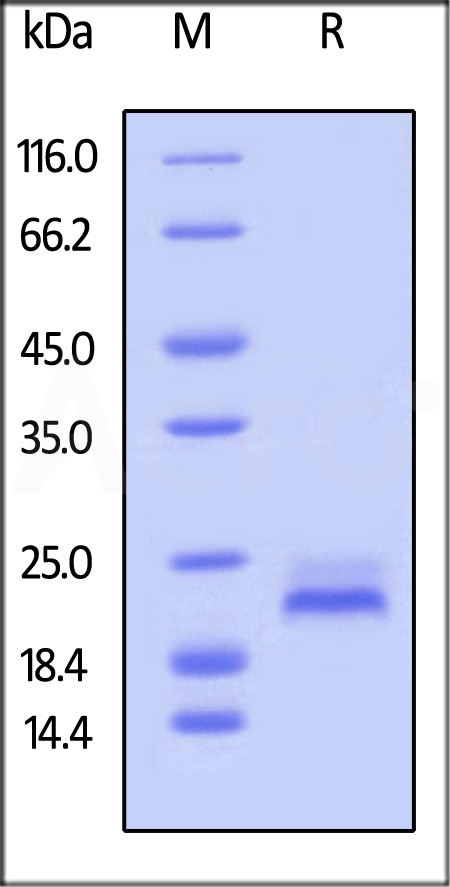
|
|
| TR2-H5255 | Human | Human TRAIL R2 / DR5 / TNFRSF10B Protein, Fc Tag |  |

|
|
| TR2-H5229 | Human | Human TRAIL R2 / DR5 / TNFRSF10B Protein, His Tag |  |

|
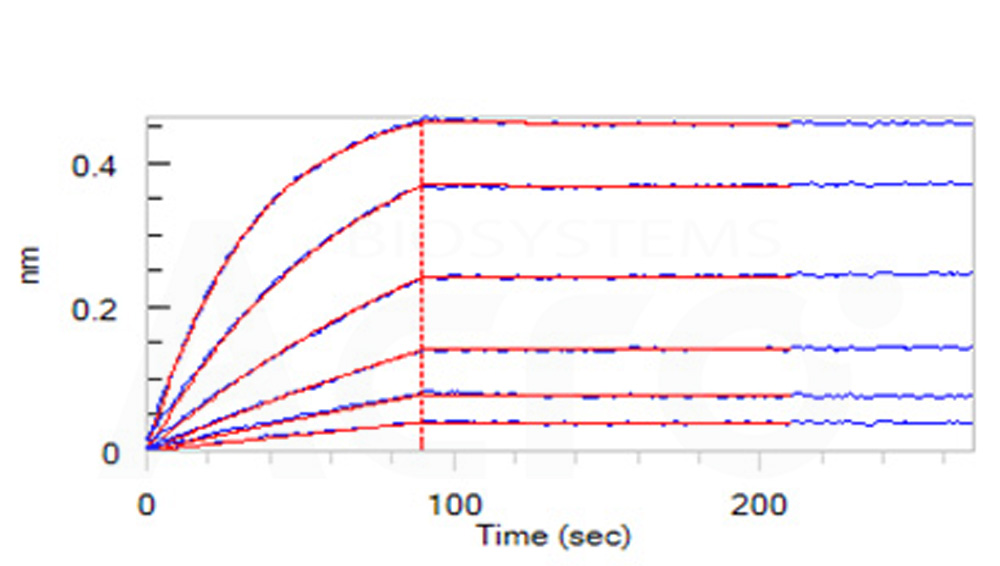
Loaded Human TRAIL R2, Fc Tag (Cat. No. TR2-H5255) on AHC Biosensor, can bind Human TRAIL, His Tag with an affinity constant of 0.146 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Aponermin | Approved | Beijing Shadong Biotech Co Ltd | 沙艾特 | Mainland China | Multiple Myeloma | Wuhan Hiteck Biological Pharma Co Ltd | 2023-11-02 | Multiple Myeloma | Details | |
| Aponermin | Approved | Beijing Shadong Biotech Co Ltd | 沙艾特 | Mainland China | Multiple Myeloma | Wuhan Hiteck Biological Pharma Co Ltd | 2023-11-02 | Multiple Myeloma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Dulanermin (Shanghai GeBaiDe) | Phase 3 Clinical | Shanghai Gebaide Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant human tumor necrosis factor-related apoptosis-inducing ligand (Shenzhen Xinpeng Biotech) | Phase 3 Clinical | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | Neoplasms | Details | |
| Conatumumab | AMG-655 | Phase 2 Clinical | Amgen Inc | Ovarian Neoplasms; Solid tumours; Rectal Neoplasms; Carcinoid Tumor; Pancreatic Neoplasms; Colonic Neoplasms; Neoplasms; Sarcoma; Colorectal Neoplasms; Lymphoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Anti-DR5 CAR-T cell therapy (Shenzhen BinDeBio) | Phase 2 Clinical | Shenzhen Bindebio Ltd | Esophageal Neoplasms; Stomach Neoplasms; Glioma; Carcinoma, Hepatocellular | Details | |
| Rilunermin alfa | SCB-313 (TRAIL-Trimer) | Phase 1 Clinical | Hydrothorax; Ascites; Peritoneal Neoplasms; Pleural Effusion, Malignant | Details | |
| BI-905711 | BI-905711 | Phase 1 Clinical | Boehringer Ingelheim Gmbh | Pancreatic Neoplasms; Cholangiocarcinoma; Gastrointestinal Neoplasms | Details |
| Recombinant anti-human DR5 monoclonal antibody (Lonn Ryonn Pharma) | CTB-006 | Phase 1 Clinical | Beijing Tongwei Shidai Shengwu Jishu Youxian Gongsi, Lonn Ryonn Pharma Ltd | Solid tumours | Details |
| Ozekibart | JCT-205; INBRX-109 | Phase 1 Clinical | Adenocarcinoma | Details | |
| Oba-01 | Oba-01; Oba01 | Phase 1 Clinical | Yantai Heyuanaidisi Biomedical Technology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| DS-8273 | DS-8273; DS-8273a | Phase 1 Clinical | Daiichi Sankyo Co Ltd | Solid tumours; Colorectal Neoplasms; Lymphoma; Melanoma | Details |
| Aplitabart | IGM-8444 | Phase 1 Clinical | Igm Biosciences Inc | Solid tumours; Chondrosarcoma; Sarcoma; Colorectal Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Dulanermin (Shanghai GeBaiDe) | Phase 3 Clinical | Shanghai Gebaide Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant human tumor necrosis factor-related apoptosis-inducing ligand (Shenzhen Xinpeng Biotech) | Phase 3 Clinical | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | Neoplasms | Details | |
| Conatumumab | AMG-655 | Phase 2 Clinical | Amgen Inc | Ovarian Neoplasms; Solid tumours; Rectal Neoplasms; Carcinoid Tumor; Pancreatic Neoplasms; Colonic Neoplasms; Neoplasms; Sarcoma; Colorectal Neoplasms; Lymphoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Anti-DR5 CAR-T cell therapy (Shenzhen BinDeBio) | Phase 2 Clinical | Shenzhen Bindebio Ltd | Esophageal Neoplasms; Stomach Neoplasms; Glioma; Carcinoma, Hepatocellular | Details | |
| Rilunermin alfa | SCB-313 (TRAIL-Trimer) | Phase 1 Clinical | Hydrothorax; Ascites; Peritoneal Neoplasms; Pleural Effusion, Malignant | Details | |
| BI-905711 | BI-905711 | Phase 1 Clinical | Boehringer Ingelheim Gmbh | Pancreatic Neoplasms; Cholangiocarcinoma; Gastrointestinal Neoplasms | Details |
| Recombinant anti-human DR5 monoclonal antibody (Lonn Ryonn Pharma) | CTB-006 | Phase 1 Clinical | Beijing Tongwei Shidai Shengwu Jishu Youxian Gongsi, Lonn Ryonn Pharma Ltd | Solid tumours | Details |
| Ozekibart | JCT-205; INBRX-109 | Phase 1 Clinical | Adenocarcinoma | Details | |
| Oba-01 | Oba-01; Oba01 | Phase 1 Clinical | Yantai Heyuanaidisi Biomedical Technology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| DS-8273 | DS-8273; DS-8273a | Phase 1 Clinical | Daiichi Sankyo Co Ltd | Solid tumours; Colorectal Neoplasms; Lymphoma; Melanoma | Details |
| Aplitabart | IGM-8444 | Phase 1 Clinical | Igm Biosciences Inc | Solid tumours; Chondrosarcoma; Sarcoma; Colorectal Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
This web search service is supported by Google Inc.
