 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| CO3-H5253 | Human | Human Complement component 3 Protein, Fc Tag (MALS verified) |  |
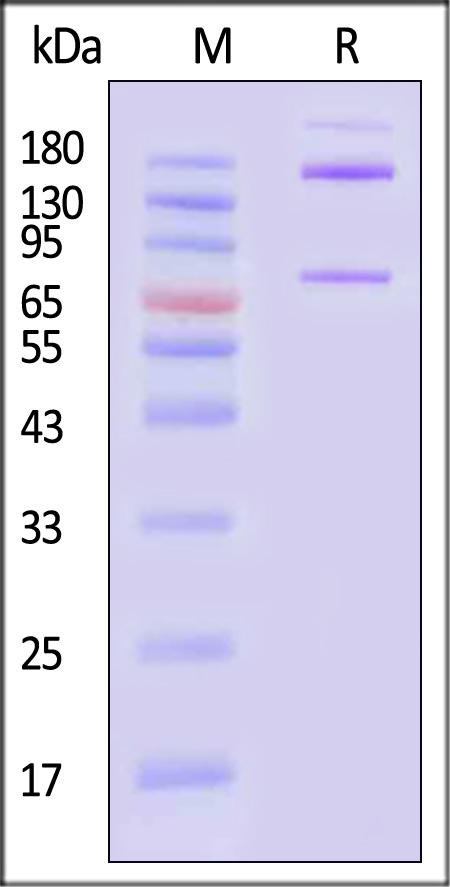
|
|
| CO3-H82E8 | Human | Biotinylated Human Complement C3 Protein, His,Avitag™ (MALS verified) |  |
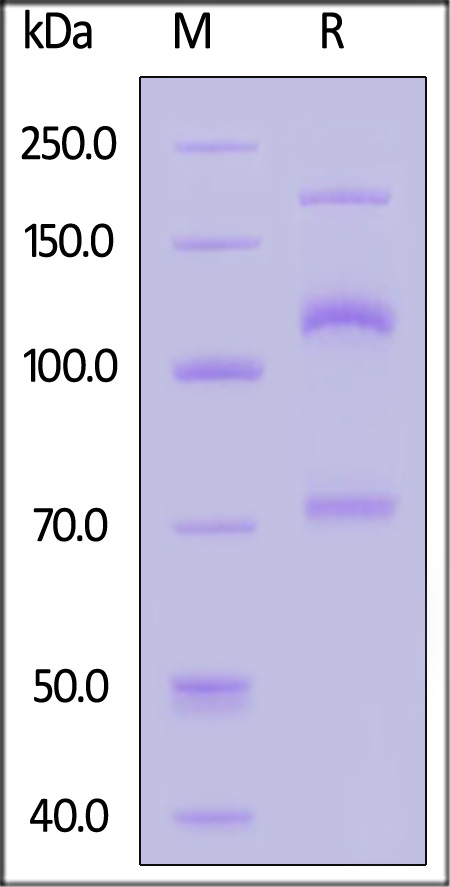
|
|
| CO3-C52H5 | Cynomolgus | Cynomolgus Complement C3 Protein, His Tag (MALS verified) |  |

|
|
| CO3-M52H4 | Mouse | Mouse Complement C3 Protein, His Tag (MALS verified) |  |

|
|
| CO3-H52H3 | Human | Human Complement C3 Protein, His Tag (MALS verified) |  |
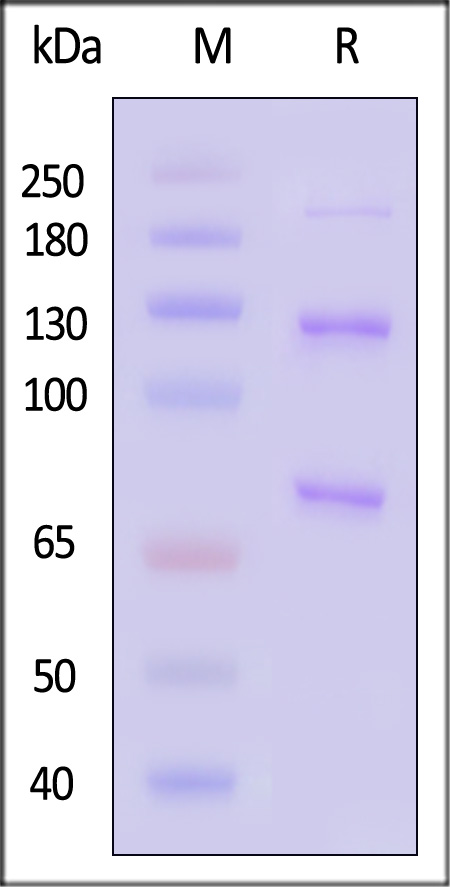
|

Immobilized Biotinylated Human Complement C3, His,Avitag (Cat. No. CO3-H82E8) at 1 μg/mL (100 μL/well)on streptavidin precoated (0.5 μg/well) plate can bind Human Anti-C3,Human IgG1 Human Kappa with a linear range of 0.1-4 ng/mL (QC tested).
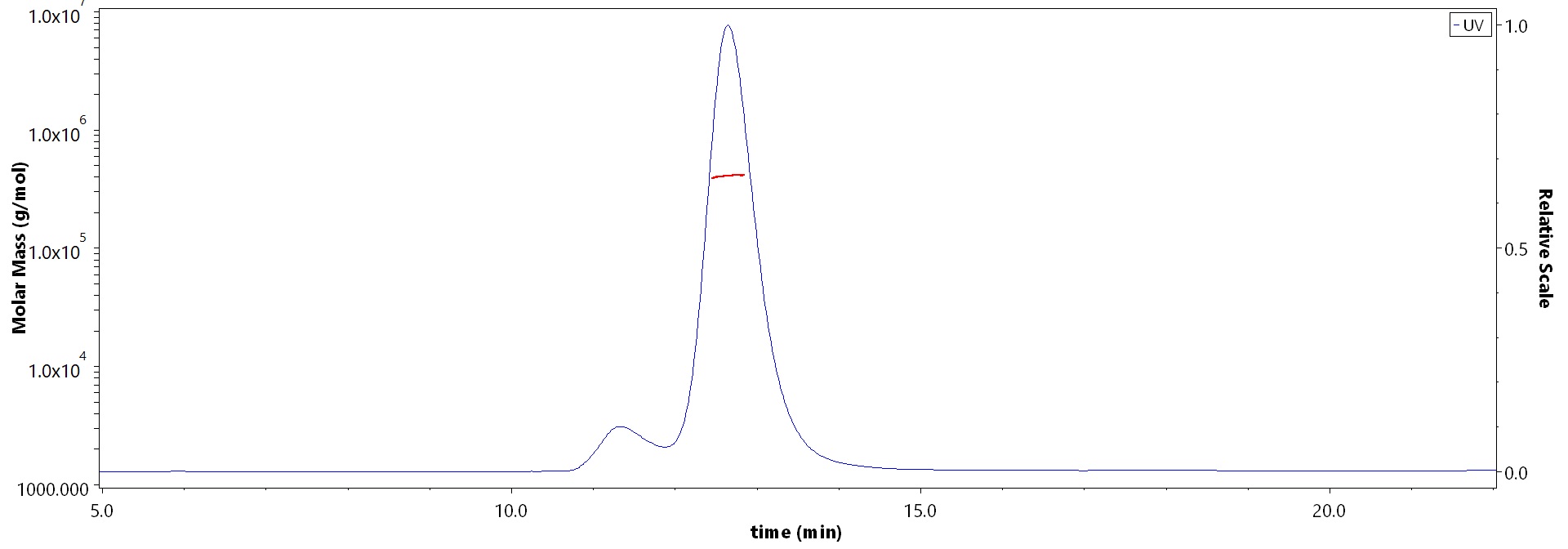
The purity of Human Complement component 3 Protein, Fc Tag (Cat. No. CO3-H5253) is more than 85% and the molecular weight of this protein is around 390-440 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Pegcetacoplan | APL-2 | Approved | Apellis Pharmaceuticals Inc | Aspaveli, Empaveli | United States | Hemoglobinuria, Paroxysmal | Apellis Pharmaceuticals Inc | 2021-05-14 | Motor Neuron Disease; Rejection of renal transplantation; Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Thrombotic Microangiopathies; Glomerulonephritis, Membranous; Lupus Nephritis; Glomerulonephritis, Membranoproliferative; Anemia, Hemolytic, Autoimmune; Geographic Atrophy; Glomerulonephritis; Amyotrophic Lateral Sclerosis; Macular Degeneration | Details |
| Pegcetacoplan | APL-2 | Approved | Apellis Pharmaceuticals Inc | Aspaveli, Empaveli | United States | Hemoglobinuria, Paroxysmal | Apellis Pharmaceuticals Inc | 2021-05-14 | Motor Neuron Disease; Rejection of renal transplantation; Hemoglobinuria, Paroxysmal; Glomerulonephritis, IGA; Thrombotic Microangiopathies; Glomerulonephritis, Membranous; Lupus Nephritis; Glomerulonephritis, Membranoproliferative; Anemia, Hemolytic, Autoimmune; Geographic Atrophy; Glomerulonephritis; Amyotrophic Lateral Sclerosis; Macular Degeneration | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Efdamrofusp alfa | IBI-302 | Phase 3 Clinical | AP Biosciences Inc | Diabetic macular oedema; Wet Macular Degeneration; Macular Degeneration; Plaque psoriasis | Details |
| APL-9 | Phase 2 Clinical | Apellis Pharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Coronavirus Infections; Severe Acute Respiratory Syndrome | Details | |
| NGM-621 | NGM-621 | Phase 2 Clinical | Ngm Biopharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Geographic Atrophy | Details |
| Compstatin 40 | AMY-101 | Phase 2 Clinical | University Of Pennsylvania | Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Gingivitis | Details |
| ARO-C3 | ARO-C3; ARO-C-3 | Phase 2 Clinical | Arrowhead Pharmaceuticals Inc | Glomerulonephritis, IGA; Glomerulonephritis | Details |
| CG-001 | CG-001 | Phase 1 Clinical | Chengdu Konjin Co Ltd | Hemoglobinuria, Paroxysmal; Autoimmune Diseases | Details |
| ADX-097 | ADX-097 | Phase 1 Clinical | Q32 Bio Inc | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Glomerulonephritis, IGA; Kidney Diseases; Nephrosis, Lipoid; Lupus Nephritis; Inflammation; Autoimmune Diseases of the Nervous System | Details |
| APL-3007 | APL-3007 | Phase 1 Clinical | Apellis Pharmaceuticals Inc | Details | |
| SGB-9768 | SGB-9768 | Phase 1 Clinical | Suzhou Sanegene Biopharmaceuticals Ltd, Sanegene Bio New Zealand Ltd | Details | |
| NM3086 | NM3086; NM-3086 | Phase 1 Clinical | NovelMed Therapeutics Inc | Hemoglobinuria, Paroxysmal | Details |
| LP-005 | LP-005; RX-001 | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Peripheral Nervous System Diseases; Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Kidney Diseases; Amyotrophic Lateral Sclerosis | Details |
| Efdamrofusp alfa | IBI-302 | Phase 3 Clinical | AP Biosciences Inc | Diabetic macular oedema; Wet Macular Degeneration; Macular Degeneration; Plaque psoriasis | Details |
| APL-9 | Phase 2 Clinical | Apellis Pharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Coronavirus Infections; Severe Acute Respiratory Syndrome | Details | |
| NGM-621 | NGM-621 | Phase 2 Clinical | Ngm Biopharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Geographic Atrophy | Details |
| Compstatin 40 | AMY-101 | Phase 2 Clinical | University Of Pennsylvania | Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Gingivitis | Details |
| ARO-C3 | ARO-C3; ARO-C-3 | Phase 2 Clinical | Arrowhead Pharmaceuticals Inc | Glomerulonephritis, IGA; Glomerulonephritis | Details |
| CG-001 | CG-001 | Phase 1 Clinical | Chengdu Konjin Co Ltd | Hemoglobinuria, Paroxysmal; Autoimmune Diseases | Details |
| ADX-097 | ADX-097 | Phase 1 Clinical | Q32 Bio Inc | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Glomerulonephritis, IGA; Kidney Diseases; Nephrosis, Lipoid; Lupus Nephritis; Inflammation; Autoimmune Diseases of the Nervous System | Details |
| APL-3007 | APL-3007 | Phase 1 Clinical | Apellis Pharmaceuticals Inc | Details | |
| SGB-9768 | SGB-9768 | Phase 1 Clinical | Suzhou Sanegene Biopharmaceuticals Ltd, Sanegene Bio New Zealand Ltd | Details | |
| NM3086 | NM3086; NM-3086 | Phase 1 Clinical | NovelMed Therapeutics Inc | Hemoglobinuria, Paroxysmal | Details |
| LP-005 | LP-005; RX-001 | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Peripheral Nervous System Diseases; Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Kidney Diseases; Amyotrophic Lateral Sclerosis | Details |
This web search service is supported by Google Inc.
