 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| AHE-H52H3 | Human | Human ACHE / Acetylcholinesterase Protein, His Tag (active enzyme) |  |
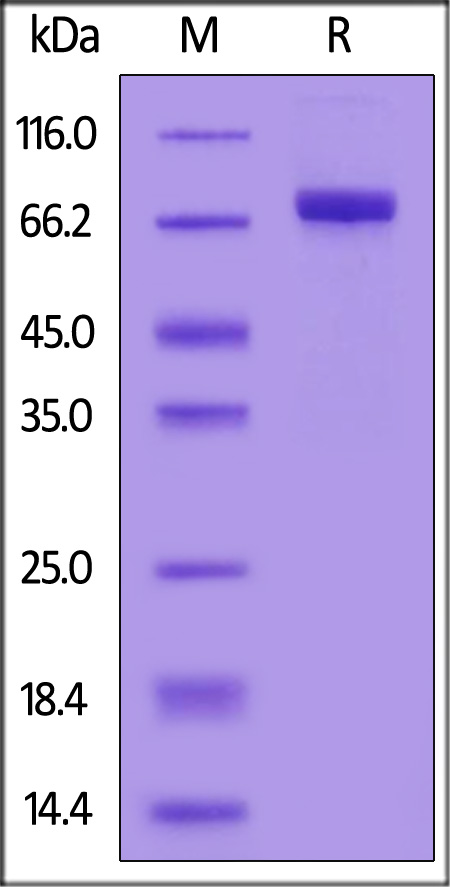
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Galantamine Hydrobromide | GP-37267; R-113675 | Approved | AverTox, Nivalin, Reminyl, Razadyne, Reminyl XL | Mainland China | Alzheimer Disease | null | 1981-01-01 | Dementia; Mental Disorders; Fasting; Subarachnoid Hemorrhage; Frontotemporal Dementia; Stress Disorders, Traumatic, Acute; Dementia, Vascular; Cognition Disorders; Cocaine-Related Disorders; Obesity; Nervous System Diseases; Lewy Body Disease; Schizophrenia; Alzheimer Disease; Cognitive Dysfunction; Tobacco Use Disorder; AIDS Dementia Complex; Vascular Diseases; Memory; Autistic Disorder; Stroke; Insulin Resistance; Brain Diseases | Details | |
| Neostigmine Bromide | Approved | Prostigmin | Mainland China | Myasthenia Gravis; Flatulence; Urinary Retention | Shanghai Sine Pharmaceutical Laboratories Co Ltd | 1995-01-01 | Myasthenia Gravis; Headache; Flatulence; Urinary Retention | Details | ||
| Metrifonate | Bay-a-9826; Bayer-L-1359 | Approved | ProMem, Memobay, Bilarcil | Schistosomiasis | Details | |||||
| Rivastigmine Tartrate | SDZ-212-713; SDZ-ENA-713; LY-30410; LY-03011; ONO-2540; ENA-713; LY 03013; ENA-713D; LY30410 | Approved | Novartis Pharma Ag | Exelon TDS, Rivastach, Exelon MR, Prometax, Exelon, 艾斯能 | EU | Parkinson Disease; Dementia; Alzheimer Disease | Novartis Europharm Ltd | 1998-05-11 | Substance-Related Disorders; Down Syndrome; Brain Injuries, Traumatic; Delirium; Neurobehavioral Manifestations; Alzheimer Disease; Parkinson Disease; Dementia; Cocaine-Related Disorders; Dementia, Vascular; Cognition Disorders | Details |
| Pyridostigmine Bromide | Ro-1-5130 | Approved | Valeant | Regonol, Mestinon | United States | Lambert-Eaton Myasthenic Syndrome | Bausch & Lomb (Singapore) Pvi Ltd | 1955-04-06 | Dyskinesias; HIV Seropositivity; Immunologic Deficiency Syndromes; Hypotension, Orthostatic; Fibromyalgia; Diabetes Mellitus; T-Lymphocytopenia, Idiopathic CD4-Positive; Fatigue Syndrome, Chronic; Parkinson Disease; Glycogen Storage Disease Type II; Constipation; Poisoning; Prostatic Neoplasms, Castration-Resistant; Coronavirus Disease 2019 (COVID-19); Postural Orthostatic Tachycardia Syndrome; Lambert-Eaton Myasthenic Syndrome; Heart Failure; Myasthenia Gravis; Primary Dysautonomias; HIV Infections | Details |
| Dimebolin hydrochloride | BRN-0622478; PF-01913539; DMB-I | Approved | Bigespas Ltd | Dimebon | Russian Federation | Rhinitis, Allergic; Dermatitis, Atopic; Hypersensitivity | null | 1983-01-01 | Liver Failure; Huntington Disease; Rhinitis, Allergic; Alzheimer Disease; Dementia; Dermatitis, Atopic; Hypersensitivity | Details |
| Donepezil Hydrochloride | DKF-310; BNAG; E-2020; E-2022; (±)-E-2020; IVL-3003; GB-5001; HZ007 | Approved | Eisai Co Ltd | Memac, Eranz, Memorit, Aricept Evess, Aricept, Aricept ODT, Aricept D, 安理申, ADLARITY | United States | Alzheimer Disease | Eisai Inc | 1996-11-25 | Cognitive Dysfunction; Autistic Disorder; Aphasia; Central Nervous System Neoplasms; Multiple Sclerosis; Breast Neoplasms; Fragile X Syndrome; Brain Neoplasms; Psychology; Alzheimer Disease; Alcoholism; Hearing Loss, Sensorineural; Dementia; Parkinson Disease; Lewy Body Disease; Wounds and Injuries; Memory Disorders; Dementia, Vascular; Neoplasm Metastasis; Cognition Disorders; Subarachnoid Hemorrhage; Autism Spectrum Disorder; Supranuclear Palsy, Progressive; Drug-Related Side Effects and Adverse Reactions; Migraine Disorders; Brain Ischemia; Diabetes Mellitus, Type 2; Depression; Down Syndrome; Osteoporosis; Neurologic Manifestations; Visual Perception; Neoplasms; Attention Deficit Disorder with Hyperactivity; Smoking Cessation; Delirium; Anxiety Disorders; Sleep Wake Disorders; Fatigue; Stroke; Radiation Injuries | Details |
| Memantine Hydrochloride/Donepezil Hydrochloride | ADS-8704; D-797/D-324; CKD 355; BPS-034; BPDO-1603; MDX-8704 | Approved | Allergan Plc | Arimenda, Namzaric | United States | Alzheimer Disease | Abbvie Inc | 2014-12-23 | Alzheimer Disease | Details |
| Huperzine A | SPN-817 | Approved | Chinese Academy Of Sciences, Mayo Clinic | 哈伯因, 双益平 | Mainland China | Alzheimer Disease | Henan Taloph Pharmaceutical Stock Co Ltd | 1994-01-01 | Schizophrenia; Brain Injuries, Traumatic; Seizures; Alzheimer Disease; Epilepsy; Learning Disabilities; Memory Disorders | Details |
| Distigmine Bromide | BC-51 | Approved | Torii Pharmaceutical Co Ltd | Ubretid | Japan | Glaucoma; Cystitis; Myasthenia Gravis; Strabismus | Torii Pharmaceutical Co Ltd | 1967-09-05 | Myasthenia Gravis; Cystitis; Glaucoma; Strabismus | Details |
| Pralidoxime Chloride | 2-PAM-chloride | Approved | Baxter International Inc | Protopam chloride | United States | Poisoning | Baxter Healthcare Corp | 1964-03-11 | Poisoning | Details |
| Pralidoxime Iodide | 2-PAMI; NSC-7760; 2-PAM Iodide | Approved | Japan | Poisoning | Sumitomo Dainippon | 1958-05-01 | Poisoning | Details | ||
| Eserine Salicylate | Approved | Synapton SR, Xalieve, Antilirium | Insulin Resistance; Pain, Postoperative; Psychotic Disorders; Glaucoma | Details | ||||||
| Neostigmine methylsulfate | Approved | Bloxiverz, Intrastigmina, Juvastigmin, Stiglyn, Metastigmin | Japan | Myasthenia Gravis; Muscle Hypotonia | kyowayakuhin Co | 1936-10-01 | Myasthenia Gravis; Flatulence; Urinary Retention; Reversal of Neuromuscular Blockade; Muscle Hypotonia | Details | ||
| Naphazoline Hydrochloride/Neostigmine methylsulfate/Glycyrrhizinate dipotassium/Chlorphenamine Maleate/Aspartic Acid/Vitamin B6 | Approved | 新乐敦 | Details | |||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Floxacillin sodium | Approved | Details | ||||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Anthralin | Approved | Details | ||||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Pralidoxime Chloride | Approved | Details | ||||||||
| Pralidoxime Chloride/Benactyzine Hydrochloride/Atropine Sulfate | Approved | Poisoning | Details | |||||||
| Naphazoline Hydrochloride/Neostigmine methylsulfate | Approved | Details | ||||||||
| Dihydrogalanthamine Hydrobromide | Approved | Mainland China | Hemiplegia; Sciatica | null | 1982-01-01 | Hemiplegia; Sciatica | Details | |||
| Securinine Nitrate | Approved | Poliomyelitis; Facial Paralysis | Details | |||||||
| Rivastigmine | Approved | Novartis Pharma Ag | Parkinson Disease; Alzheimer Disease | Details | ||||||
| Glycopyrronium bromide/Neostigmine metilsulfate | Approved | Sintetica Gmbh | Novistig | Sintetica Gmbh | 2020-07-29 | Drug Overdose; Reversal of Neuromuscular Blockade; Muscle Hypotonia; Neuromuscular Diseases | Details | |||
| Atropine/Pralidoxime Chloride | Approved | Meridian Medical Technologies Inc | DUODOTE | United States | Poisoning; Organophosphate Poisoning | Meridian Medical Technologies LLC | 2002-01-17 | Drug-Related Side Effects and Adverse Reactions; Organophosphate Poisoning; Poisoning; Drug intoxications | Details | |
| Acotiamide Hydrochloride Hydrate | Z-338; YM-443 | Approved | Zeria | Acofide | Japan | Dyspepsia | null | 2013-03-25 | Dyspepsia; Nausea | Details |
| Galantamine Hydrobromide | GP-37267; R-113675 | Approved | AverTox, Nivalin, Reminyl, Razadyne, Reminyl XL | Mainland China | Alzheimer Disease | null | 1981-01-01 | Dementia; Mental Disorders; Fasting; Subarachnoid Hemorrhage; Frontotemporal Dementia; Stress Disorders, Traumatic, Acute; Dementia, Vascular; Cognition Disorders; Cocaine-Related Disorders; Obesity; Nervous System Diseases; Lewy Body Disease; Schizophrenia; Alzheimer Disease; Cognitive Dysfunction; Tobacco Use Disorder; AIDS Dementia Complex; Vascular Diseases; Memory; Autistic Disorder; Stroke; Insulin Resistance; Brain Diseases | Details | |
| Neostigmine Bromide | Approved | Prostigmin | Mainland China | Myasthenia Gravis; Flatulence; Urinary Retention | Shanghai Sine Pharmaceutical Laboratories Co Ltd | 1995-01-01 | Myasthenia Gravis; Headache; Flatulence; Urinary Retention | Details | ||
| Metrifonate | Bay-a-9826; Bayer-L-1359 | Approved | ProMem, Memobay, Bilarcil | Schistosomiasis | Details | |||||
| Rivastigmine Tartrate | SDZ-212-713; SDZ-ENA-713; LY-30410; LY-03011; ONO-2540; ENA-713; LY 03013; ENA-713D; LY30410 | Approved | Novartis Pharma Ag | Exelon TDS, Rivastach, Exelon MR, Prometax, Exelon, 艾斯能 | EU | Parkinson Disease; Dementia; Alzheimer Disease | Novartis Europharm Ltd | 1998-05-11 | Substance-Related Disorders; Down Syndrome; Brain Injuries, Traumatic; Delirium; Neurobehavioral Manifestations; Alzheimer Disease; Parkinson Disease; Dementia; Cocaine-Related Disorders; Dementia, Vascular; Cognition Disorders | Details |
| Pyridostigmine Bromide | Ro-1-5130 | Approved | Valeant | Regonol, Mestinon | United States | Lambert-Eaton Myasthenic Syndrome | Bausch & Lomb (Singapore) Pvi Ltd | 1955-04-06 | Dyskinesias; HIV Seropositivity; Immunologic Deficiency Syndromes; Hypotension, Orthostatic; Fibromyalgia; Diabetes Mellitus; T-Lymphocytopenia, Idiopathic CD4-Positive; Fatigue Syndrome, Chronic; Parkinson Disease; Glycogen Storage Disease Type II; Constipation; Poisoning; Prostatic Neoplasms, Castration-Resistant; Coronavirus Disease 2019 (COVID-19); Postural Orthostatic Tachycardia Syndrome; Lambert-Eaton Myasthenic Syndrome; Heart Failure; Myasthenia Gravis; Primary Dysautonomias; HIV Infections | Details |
| Dimebolin hydrochloride | BRN-0622478; PF-01913539; DMB-I | Approved | Bigespas Ltd | Dimebon | Russian Federation | Rhinitis, Allergic; Dermatitis, Atopic; Hypersensitivity | null | 1983-01-01 | Liver Failure; Huntington Disease; Rhinitis, Allergic; Alzheimer Disease; Dementia; Dermatitis, Atopic; Hypersensitivity | Details |
| Donepezil Hydrochloride | DKF-310; BNAG; E-2020; E-2022; (±)-E-2020; IVL-3003; GB-5001; HZ007 | Approved | Eisai Co Ltd | Memac, Eranz, Memorit, Aricept Evess, Aricept, Aricept ODT, Aricept D, 安理申, ADLARITY | United States | Alzheimer Disease | Eisai Inc | 1996-11-25 | Cognitive Dysfunction; Autistic Disorder; Aphasia; Central Nervous System Neoplasms; Multiple Sclerosis; Breast Neoplasms; Fragile X Syndrome; Brain Neoplasms; Psychology; Alzheimer Disease; Alcoholism; Hearing Loss, Sensorineural; Dementia; Parkinson Disease; Lewy Body Disease; Wounds and Injuries; Memory Disorders; Dementia, Vascular; Neoplasm Metastasis; Cognition Disorders; Subarachnoid Hemorrhage; Autism Spectrum Disorder; Supranuclear Palsy, Progressive; Drug-Related Side Effects and Adverse Reactions; Migraine Disorders; Brain Ischemia; Diabetes Mellitus, Type 2; Depression; Down Syndrome; Osteoporosis; Neurologic Manifestations; Visual Perception; Neoplasms; Attention Deficit Disorder with Hyperactivity; Smoking Cessation; Delirium; Anxiety Disorders; Sleep Wake Disorders; Fatigue; Stroke; Radiation Injuries | Details |
| Memantine Hydrochloride/Donepezil Hydrochloride | ADS-8704; D-797/D-324; CKD 355; BPS-034; BPDO-1603; MDX-8704 | Approved | Allergan Plc | Arimenda, Namzaric | United States | Alzheimer Disease | Abbvie Inc | 2014-12-23 | Alzheimer Disease | Details |
| Huperzine A | SPN-817 | Approved | Chinese Academy Of Sciences, Mayo Clinic | 哈伯因, 双益平 | Mainland China | Alzheimer Disease | Henan Taloph Pharmaceutical Stock Co Ltd | 1994-01-01 | Schizophrenia; Brain Injuries, Traumatic; Seizures; Alzheimer Disease; Epilepsy; Learning Disabilities; Memory Disorders | Details |
| Distigmine Bromide | BC-51 | Approved | Torii Pharmaceutical Co Ltd | Ubretid | Japan | Glaucoma; Cystitis; Myasthenia Gravis; Strabismus | Torii Pharmaceutical Co Ltd | 1967-09-05 | Myasthenia Gravis; Cystitis; Glaucoma; Strabismus | Details |
| Pralidoxime Chloride | 2-PAM-chloride | Approved | Baxter International Inc | Protopam chloride | United States | Poisoning | Baxter Healthcare Corp | 1964-03-11 | Poisoning | Details |
| Pralidoxime Iodide | 2-PAMI; NSC-7760; 2-PAM Iodide | Approved | Japan | Poisoning | Sumitomo Dainippon | 1958-05-01 | Poisoning | Details | ||
| Eserine Salicylate | Approved | Synapton SR, Xalieve, Antilirium | Insulin Resistance; Pain, Postoperative; Psychotic Disorders; Glaucoma | Details | ||||||
| Neostigmine methylsulfate | Approved | Bloxiverz, Intrastigmina, Juvastigmin, Stiglyn, Metastigmin | Japan | Myasthenia Gravis; Muscle Hypotonia | kyowayakuhin Co | 1936-10-01 | Myasthenia Gravis; Flatulence; Urinary Retention; Reversal of Neuromuscular Blockade; Muscle Hypotonia | Details | ||
| Naphazoline Hydrochloride/Neostigmine methylsulfate/Glycyrrhizinate dipotassium/Chlorphenamine Maleate/Aspartic Acid/Vitamin B6 | Approved | 新乐敦 | Details | |||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Floxacillin sodium | Approved | Details | ||||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Anthralin | Approved | Details | ||||||||
| Dextrose/Sodium Chloride/Potassium Chloride/Pralidoxime Chloride | Approved | Details | ||||||||
| Pralidoxime Chloride/Benactyzine Hydrochloride/Atropine Sulfate | Approved | Poisoning | Details | |||||||
| Naphazoline Hydrochloride/Neostigmine methylsulfate | Approved | Details | ||||||||
| Dihydrogalanthamine Hydrobromide | Approved | Mainland China | Hemiplegia; Sciatica | null | 1982-01-01 | Hemiplegia; Sciatica | Details | |||
| Securinine Nitrate | Approved | Poliomyelitis; Facial Paralysis | Details | |||||||
| Rivastigmine | Approved | Novartis Pharma Ag | Parkinson Disease; Alzheimer Disease | Details | ||||||
| Glycopyrronium bromide/Neostigmine metilsulfate | Approved | Sintetica Gmbh | Novistig | Sintetica Gmbh | 2020-07-29 | Drug Overdose; Reversal of Neuromuscular Blockade; Muscle Hypotonia; Neuromuscular Diseases | Details | |||
| Atropine/Pralidoxime Chloride | Approved | Meridian Medical Technologies Inc | DUODOTE | United States | Poisoning; Organophosphate Poisoning | Meridian Medical Technologies LLC | 2002-01-17 | Drug-Related Side Effects and Adverse Reactions; Organophosphate Poisoning; Poisoning; Drug intoxications | Details | |
| Acotiamide Hydrochloride Hydrate | Z-338; YM-443 | Approved | Zeria | Acofide | Japan | Dyspepsia | null | 2013-03-25 | Dyspepsia; Nausea | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Octahydroaminoacridine succinate | 华洋-001 | Phase 3 Clinical | Changchun Huayang High-Tech Co Ltd, Jiangsu Sheneryang High-Tech Co Ltd | Alzheimer Disease | Details |
| Donepezil/Solifenacin | CPC-201 | Phase 3 Clinical | Cipla Inc, Allergan Plc | Urologic Diseases; Alzheimer Disease; Dementia | Details |
| Botulinum toxin (CKD Bio) | CKDB-501B; CKDB-501-B; CKDB-501A; CKDB-501-A; CKDB-501 | Phase 3 Clinical | CKD Bio Corp | Nasolabial fold wrinkles; Upper and lower limb spasticity | Details |
| BR-4001 | BNT-002; BR-4001 | Phase 3 Clinical | Boryung Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| AGN-151586 | AGN-151586 | Phase 3 Clinical | Allergan Plc | Nasolabial fold wrinkles | Details |
| Blarcamesine/Donepezil Hydrochloride | Phase 2 Clinical | Anavex Life Sciences Corp | Alzheimer Disease | Details | |
| Ondansetron Hydrochloride/Pyridostigmine Bromide | GTB-004 | Phase 2 Clinical | Gt Biopharma Inc | Myasthenia Gravis | Details |
| Pregnenolone | NBI-113 | Phase 2 Clinical | Neurocrine Biosciences Inc | Depression; Anxiety; Schizophrenia; Autism Spectrum Disorder; Menopausal symptoms; Depressive Disorder, Major; Autistic Disorder; Alcoholism; Stress Disorders, Post-Traumatic; Cocaine-Related Disorders; Low Back Pain | Details |
| Fluoropezil | DC-20; DC-511020 | Phase 2 Clinical | Jiangsu Kanion Pharmaceutical Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Alzheimer Disease | Details |
| Pyridostigmine | K-127 | Phase 2 Clinical | Kashiv BioSciences LLC | Musculoskeletal Diseases; Blepharoptosis; Urination Disorders; Fatigue Syndrome, Chronic | Details |
| Huperizine-A (Biscayne Pharmaceuticals) | INS-001; BIS-001-ER; XEL-001-HG; XEL-001-HP; Hup-A; BIS-001 | Phase 2 Clinical | Harvard University | Seizures; Epilepsy, Complex Partial | Details |
| EX-039 | EX-039 | Phase 2 Clinical | Excelsior Corp | Alzheimer Disease | Details |
| donepezil (Vyant Bio) | VYNT-0126 | Phase 2 Clinical | Vyant Bio Inc | Rett Syndrome | Details |
| YIV-906 | PHY-906; KD-018 | Phase 2 Clinical | Phytoceutica Inc | Liver Neoplasms; Rectal Neoplasms; Pancreatic Neoplasms; Diarrhea; Hepatitis B; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Benzgalantamine | GLN-1062; ALPHA-1062; ALPHA-1062IN | Phase 2 Clinical | Galantos Pharma GmbH | Brain Injuries, Traumatic; Alzheimer Disease | Details |
| AD-35 | AD-35 | Phase 2 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| Edrophonium Chloride | Phase 1 Clinical | Teligent | Myasthenia Gravis | Details | |
| 18F-flubatine | 18F-flubatine; norchloro-fluoroepibatidine-[18F]; flubatine-[18F]; Fluorine-18-flubatine | Phase 1 Clinical | Helmholtz-Zentrum Dresden-Rossendorf | Alzheimer Disease | Details |
| Donepezil Hydrochloride/Mefloquine Hydrochloride | THN-201 | Phase 1 Clinical | Theranexus | Cognitive Dysfunction; Alzheimer Disease | Details |
| Mecripyrine Hydrochloride | SCR-1693; Y-1A; Y-1 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Yantai Yene Pharma Co Ltd, NeuroDawn Pharmaceutical Co Ltd | Brain Neoplasms; Alzheimer Disease | Details |
| Methanesulfonyl fluoride | SNX-001 | Phase 1 Clinical | Senexta | Alzheimer Disease | Details |
| Galantamine/Metformin | RJx-01 | Phase 1 Clinical | Rejuvenate Biomed NV | Muscular Atrophy | Details |
| Donepezil transdermal patch(Dong-A ST) | DA-5207 | Phase 1 Clinical | Dong-A ST Co Ltd | Details | |
| HHT-201 | HHT-201 | Phase 1 Clinical | Zhejiang Huahai Pharmaceutical Co Ltd | Hypertension; Alzheimer Disease | Details |
| Octahydroaminoacridine succinate | 华洋-001 | Phase 3 Clinical | Changchun Huayang High-Tech Co Ltd, Jiangsu Sheneryang High-Tech Co Ltd | Alzheimer Disease | Details |
| Donepezil/Solifenacin | CPC-201 | Phase 3 Clinical | Cipla Inc, Allergan Plc | Urologic Diseases; Alzheimer Disease; Dementia | Details |
| Botulinum toxin (CKD Bio) | CKDB-501B; CKDB-501-B; CKDB-501A; CKDB-501-A; CKDB-501 | Phase 3 Clinical | CKD Bio Corp | Nasolabial fold wrinkles; Upper and lower limb spasticity | Details |
| BR-4001 | BNT-002; BR-4001 | Phase 3 Clinical | Boryung Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| AGN-151586 | AGN-151586 | Phase 3 Clinical | Allergan Plc | Nasolabial fold wrinkles | Details |
| Blarcamesine/Donepezil Hydrochloride | Phase 2 Clinical | Anavex Life Sciences Corp | Alzheimer Disease | Details | |
| Ondansetron Hydrochloride/Pyridostigmine Bromide | GTB-004 | Phase 2 Clinical | Gt Biopharma Inc | Myasthenia Gravis | Details |
| Pregnenolone | NBI-113 | Phase 2 Clinical | Neurocrine Biosciences Inc | Depression; Anxiety; Schizophrenia; Autism Spectrum Disorder; Menopausal symptoms; Depressive Disorder, Major; Autistic Disorder; Alcoholism; Stress Disorders, Post-Traumatic; Cocaine-Related Disorders; Low Back Pain | Details |
| Fluoropezil | DC-20; DC-511020 | Phase 2 Clinical | Jiangsu Kanion Pharmaceutical Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Alzheimer Disease | Details |
| Pyridostigmine | K-127 | Phase 2 Clinical | Kashiv BioSciences LLC | Musculoskeletal Diseases; Blepharoptosis; Urination Disorders; Fatigue Syndrome, Chronic | Details |
| Huperizine-A (Biscayne Pharmaceuticals) | INS-001; BIS-001-ER; XEL-001-HG; XEL-001-HP; Hup-A; BIS-001 | Phase 2 Clinical | Harvard University | Seizures; Epilepsy, Complex Partial | Details |
| EX-039 | EX-039 | Phase 2 Clinical | Excelsior Corp | Alzheimer Disease | Details |
| donepezil (Vyant Bio) | VYNT-0126 | Phase 2 Clinical | Vyant Bio Inc | Rett Syndrome | Details |
| YIV-906 | PHY-906; KD-018 | Phase 2 Clinical | Phytoceutica Inc | Liver Neoplasms; Rectal Neoplasms; Pancreatic Neoplasms; Diarrhea; Hepatitis B; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Benzgalantamine | GLN-1062; ALPHA-1062; ALPHA-1062IN | Phase 2 Clinical | Galantos Pharma GmbH | Brain Injuries, Traumatic; Alzheimer Disease | Details |
| AD-35 | AD-35 | Phase 2 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| Edrophonium Chloride | Phase 1 Clinical | Teligent | Myasthenia Gravis | Details | |
| 18F-flubatine | 18F-flubatine; norchloro-fluoroepibatidine-[18F]; flubatine-[18F]; Fluorine-18-flubatine | Phase 1 Clinical | Helmholtz-Zentrum Dresden-Rossendorf | Alzheimer Disease | Details |
| Donepezil Hydrochloride/Mefloquine Hydrochloride | THN-201 | Phase 1 Clinical | Theranexus | Cognitive Dysfunction; Alzheimer Disease | Details |
| Mecripyrine Hydrochloride | SCR-1693; Y-1A; Y-1 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Yantai Yene Pharma Co Ltd, NeuroDawn Pharmaceutical Co Ltd | Brain Neoplasms; Alzheimer Disease | Details |
| Methanesulfonyl fluoride | SNX-001 | Phase 1 Clinical | Senexta | Alzheimer Disease | Details |
| Galantamine/Metformin | RJx-01 | Phase 1 Clinical | Rejuvenate Biomed NV | Muscular Atrophy | Details |
| Donepezil transdermal patch(Dong-A ST) | DA-5207 | Phase 1 Clinical | Dong-A ST Co Ltd | Details | |
| HHT-201 | HHT-201 | Phase 1 Clinical | Zhejiang Huahai Pharmaceutical Co Ltd | Hypertension; Alzheimer Disease | Details |
This web search service is supported by Google Inc.
