
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| FG3-H52H3 | Human | Human FGF-23 (R179Q) Protein, His Tag |  |
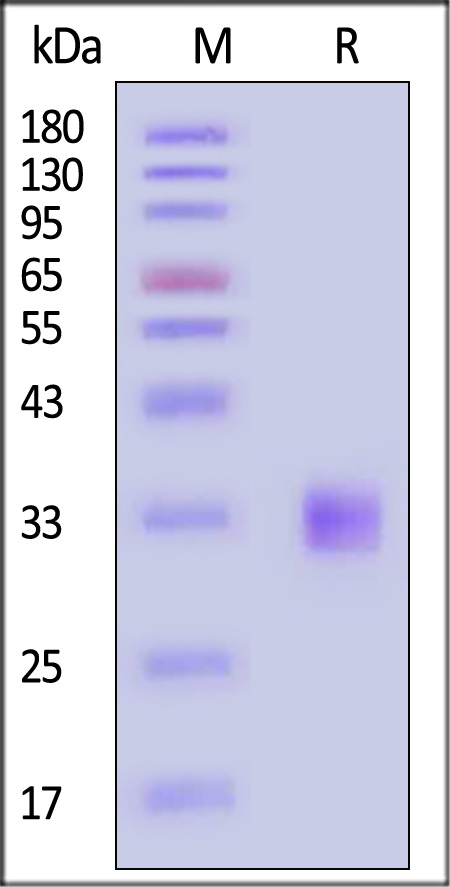
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Burosumab | KRN-23; UX-023 | Approved | Kyowa Hakko Kirin Co Ltd | Crysvita | EU | Hypophosphatemia, Familial | Kyowa Kirin Holdings Bv | 2018-02-19 | Hypophosphatemia; Fibrous Dysplasia of Bone; Hypophosphatemia, Familial; Genetic Diseases, X-Linked; Osteomalacia; Nevus, Sebaceous of Jadassohn; Familial Hypophosphatemic Rickets | Details |
| Burosumab | KRN-23; UX-023 | Approved | Kyowa Hakko Kirin Co Ltd | Crysvita | EU | Hypophosphatemia, Familial | Kyowa Kirin Holdings Bv | 2018-02-19 | Hypophosphatemia; Fibrous Dysplasia of Bone; Hypophosphatemia, Familial; Genetic Diseases, X-Linked; Osteomalacia; Nevus, Sebaceous of Jadassohn; Familial Hypophosphatemic Rickets | Details |
This web search service is supported by Google Inc.








