Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Variant Boosters, Bivalent and Polyvalent Vaccines… Who’s Going to Break the Stalemate with COVID-19? 
Vaccines play an integral role in controlling the spread of SARS-CoV-2. However, despite the extensively vaccinated population, cases and reinfections are continuously rising across the world. In particular, the emergence of the Omicron and its subvariants have triggered waves of the COVID pandemic. This phenomenon is elucidated in a recent study published in medRxiv, where populations vaccinated using current mRNA vaccines are 20 to 130 times less sensitive to Omicron mutations. As newer, more infectious variants begin to challenge and escape our existing immunity, people are becoming increasingly worried about the efficacy of current vaccines.
As identified by many tests, the frequent occurrence of new, highly transmissible variants substantially weakens protection against infection over time. To combat the diminishing vaccine efficacy, booster shots are often recommended. However, a balance needs to be struck between severe illness prevention and the recommended frequency of booster shots.
This is what the VRBPAC (Vaccine and Related Biological Products Advisory Committee) seeks to achieve. Similarly, experts reporting to the FDA (Food and Drug Administration) suggested that the new generation of COVID-19 vaccines should target the emergent strains and be provided annually. This reinforces the notion that vaccine manufacturers should continue to develop next-generation COVID vaccines against Omicron and any future strains.
Despite BA.1-based booster shots performing well against other strains, there is an increasing amount of uncertainty on the length of effectiveness. Since BA.5, the most contagious version of the virus thus far, has become the dominant strain, the FDA has recommended vaccine manufacturers to focus on developing a bivalent vaccine containing BA.4/5 components.
Aligning with the FDA recommendations, Moderna announced new clinical data on its Omicron-containing bivalent COVID booster candidate, mRNA-1273.214. Compared with the original mRNA-1273 vaccine, a booster dose of the new bivalent candidate induced 1.75 times more neutralizing antibody response against the Omicron-variant (Figure 1). Another bivalent COVID booster, mRNA-1273.222, is also in their pipeline and is expected to provide immunity from the BA.4/5 variant.

FIGURE 1: Comparison between mRNA-1273.214 and mRNA-1273
(From Modern)
Similarly, Pfizer and BioNTech announced positive data regarding their bivalent vaccine on its safety, tolerability, and immunogenicity. The bivalent vaccine contains a combination of their candidate targeting the spike protein of the Omicron BA.1 VOC with their original COVID-19 vaccine. Data from Phase 2 and 3 clinical trials also highlighted a substantially higher immune response against Omicron BA.1 in comparison to their current COVID-19 vaccine. (Figure 2) Additionally, the SARS-CoV-2 live virus neutralization assay tested on sera from participants over 56 years of age or older showed that sera could still efficiently neutralize BA.4/5 despite a 3-fold lower titer result than BA.1.

Omicron-containing Modified Variant Vaccines as 4th Dose Elicit Improved Omicron Neutralization Response (From Pfizer and BioNTech)
As new variants of SARS-CoV-2 continue to appear, it is likely for the virus to coexist with humans for the time being. Currently, many of the next-generation mutant or bivalent vaccines are developed against the current dominant variant as booster shots. Although booster shots offer a certain amount of protection against infection, it is unfeasible rely on them as a permanent solution. We expect other types of new vaccines, such as polyvalent, pan-coronavirus, or all-in-one vaccines, to be developed in the near future. These will likely attempt to achieve comprehensive protection that includes future strains of SARS-CoV-2 and other novel coronaviruses. Despite the significant challenges remaining, it is critical for scientists and vaccine manufacturers to continue their efforts in fighting against COVID-19 and future pandemics.
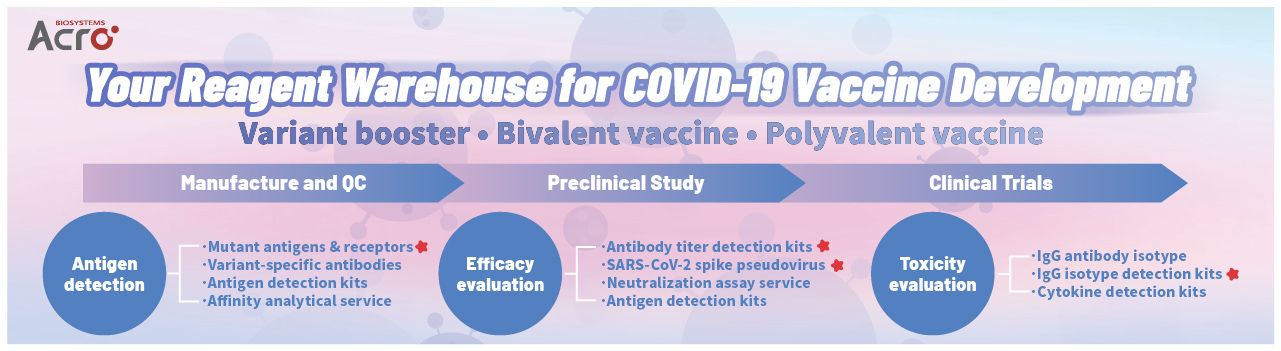
At ACROBiosystems, we remain committed to contributing to the efforts against COVID-19. To support the efforts of vaccine manufacturers, we offer and continuously update a series of core reagents as a comprehensive solution to develop mutant-based and bivalent vaccines.
This web search service is supported by Google Inc.