Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > An effective tool to accelerate the COVID-19 neutralizing antibody screening With confirmed COVID-19 cases worldwide surpassing 6 million, scientists are pushing forward with efforts to develop vaccines and treatments to slow the pandemic and lessen the disease’s damage. There are hundreds of projects around the world focused on the development of a vaccine for the COVID-19. As of May 31, eight candidate vaccines were being tested in clinical trials. [1] But the more likely availability of the vaccine is next year.
On the other hand, the development cycle of neutralizing antibodies is relatively short. That's why many experts believe the efforts of the companies developing antibody drugs are so vital. What makes antibodies unique, compared to vaccines or antiviral drugs, is their potential to both treat and protect against viral infections. With the advantage of high specificity and easy to scale up, the neutralizing antibody development is drawing as much attention around the world.
Recently, Sunney Xie’s group published their work about the screening of the neutralizing antibodies against SARS-CoV-2 in top journal Cell. Let’s walk you through their work then.
They hypothesized that enriched B cell clonotypes would more likely yield high-affinity SARS-CoV-2 binding and neutralizing antibodies. So, they first did a high-throughput sequencing of single B cells from convalescent patients. Unfortunately, they only got one RBD-binding mAb with a weak virus neutralization ability out of their first try. To optimize the method, they used RBD and S protein pre-coupled magnetic beads to enrich the RBD binding B cells (Fig. 1). Based on a set of criteria, a total of 169 ideal candidates were selected. After further screening using ELISA and SPR, 149 S-binding mAbs were identified, among which 70 mAbs bind to the RBD.
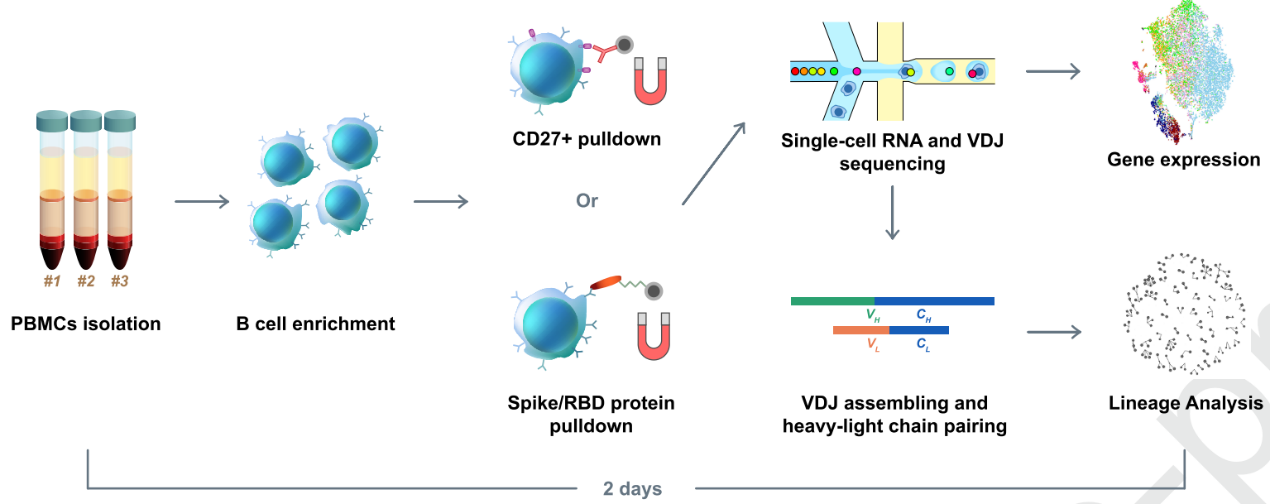
Fig.1 Illustration of RBD binding B cells enrichment using RBD and S protein pre-coupled magnetic beads
They further screened all ELISA-positive mAbs for neutralizing ability using a SARS-CoV-2 pseudovirus system, and found that among all the neutralizing mAbs, seven of them showed potent neutralization ability with an IC50 lower than 0.05 μg/mL (Fig. 2A). To validate their neutralization potential against the authentic virus, they performed the plaque reduction neutralization test and found that BD-368-2 displayed the highest potency against the authentic virus with an IC50 of 15 ng/mL (Fig. 2B).
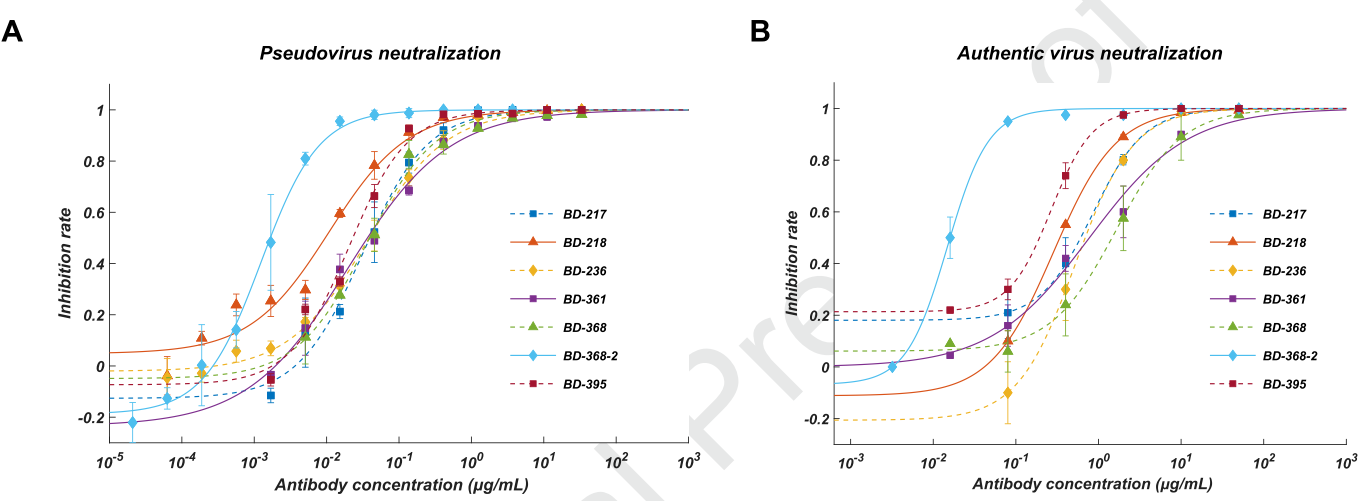
Fig. 2 A. Pseudovirus neutralization result; B. Authentic virus neutralization result.
To evaluate whether the identified neutralizing mAbs could serve as therapeutic interventions and prophylactic protection against SARS-COV-2 in vivo, they tested the neutralization efficacy of BD-368-2 on hACE2 transgenic mice infected with SARS-CoV-2. It turned out that BD-368-2 exhibited high therapeutic and prophylactic efficacy in vivo (Fig. 3).
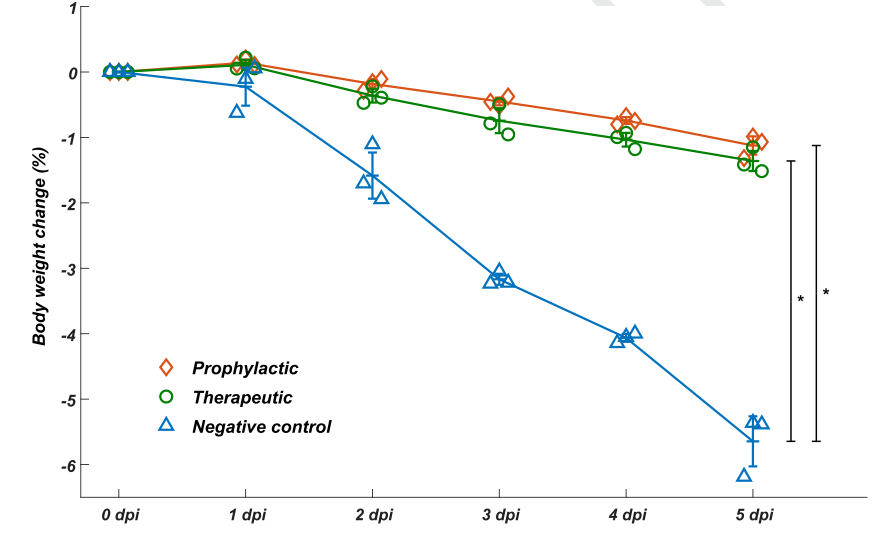
Fig. 3 BD-368-2 exhibits high therapeutic and prophylactic efficacy in vivo
Based on their Cry-EM results below (Fig. 4), we can see that the S ectodomain adopts an asymmetric conformation with the RBD in one protomer (mol A) adopting an “up” position, whereas the other two RBDs (mol B and C) adopt “down” positions. In this 3D reconstruction, a single BD23-Fab is observed per S trimer, and it binds the “down” RBD in protomer B. Only the heavy chain variable domain of BD-23 is involved in binding to the RBD.
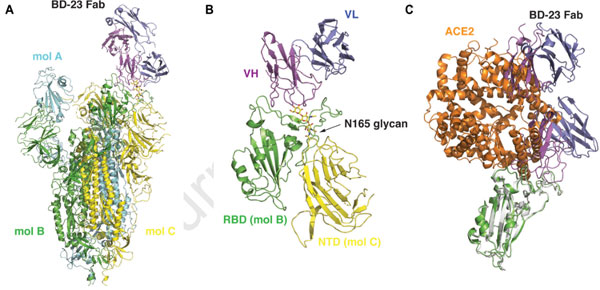
Fig. 4 3D reconstruction results of Cryo-EM.
Bioinformatic-based selection approaches were further utilized in their study and they found that mAbs that neutralizing antibodies sharing a highly similar CDR3H structure with SARS-CoV showed a high percentage of high neutralization potency for SARS-CoV-2. In other words, structure analysis can help to increase the efficiency of the neutralizing antibody screening.
A highlight of this study is that they used the RBD and S protein pre-coupled magnetic beads to enrich the B cell clonotypes. According to the interview of Sunney Xie, the corresponding author of the cell paper, the pre-coupled magnetic beads can help to increase the efficiency of B cell enrichment by 20-fold. [3]
To fill the gap in the market, ACROBiosystems has developed high-quality S1 protein and RBD protein pre-coupled magnetic beads. These pre-coupled magnetic beads will bring great convenience with minimum non-specific binding and validated protocols. This ready-to-use product could greatly save your time and eventually accelerating the therapeutic antibody development.
>>>Quantification of the pre-coupled RBD protein
Static adsorption experiments show that RBD protein-coupled magnetic beads can well capture the anti-SARS-CoV-2 S1 antibody, and the RBD load is greater than 40μg / mg magnetic beads.
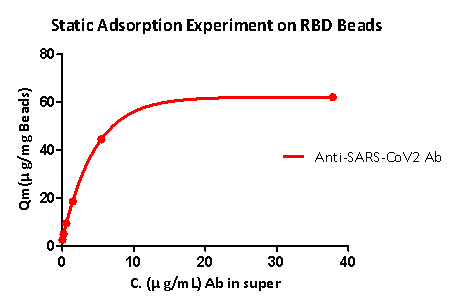
Fig 5. Static adsorption experiment on RBD beads
>>>Binding Test of RBD pre-couple magnetic beads
Strict QC tests were carried out to these pre-coupled beads. The brief process is as below: 0.1 mg beads were added to a tube. The supernatant was removed after washing. 100 μL of the antibody or ACE2 protein (10 μg / mL ~ 0.039 μg / mL) was added to the beads for 1-hour incubation. A fluorescent-labeled secondary antibody was used for detection.
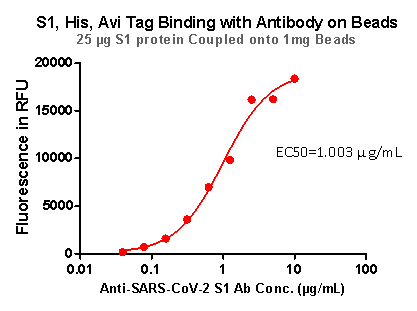
Fig 6. S1,His,Avi Tag Binding with Antibody on beads
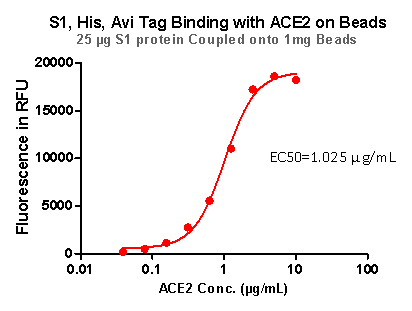
Fig 7. S1,His,Avi Tag Binding with ACE2 on Beads
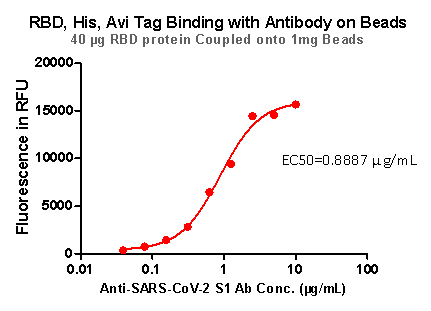
Fig 8. RBD,His,Avi Tag Binding with Antibody on beads
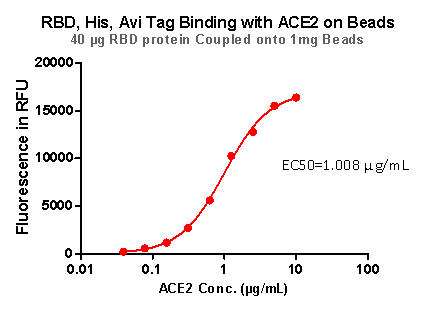
Fig 9. RBD,His,Avi Tag Binding with ACE2 on beads
Pre-coupled magnetic beads can be used for immunocapture, sample enrichment, screening as well as biopanning. What matters to the performance of the pre-coupled beads product is the quality of both the microsphere and the coupled protein. ACROBiosystems is committed to developing better and high-quality products for the pharmaceutical industry.
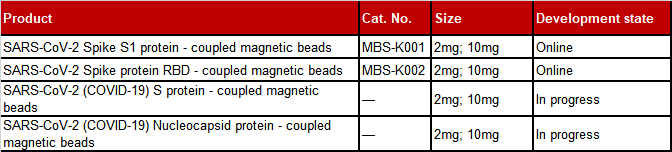
Product List
>>> Check all SARS-CoV-2 proteins and inhibitory kits.
Reference:
1. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
2. Cao, Y., et al., Potent neutralizing antibodies against SARS-CoV-2 identified by high-through put single-cell sequencing of convalescent patients’ B cells, Cell (2020), doi: https://doi.org/10.1016/j.cell.2020.05.025.
3. https://finance.sina.com.cn/wm/2020-05-21/doc-iirczymk2863827.shtml?cre=tianyi&mod=pcpager_focus&loc=1&r=9&rfunc=61&tj=none&tr=9
This web search service is supported by Google Inc.