Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Molecular test, also known as nucleic acid amplification, is recommended by the FDA to diagnose the COVID-19 virus. Nucleic acid amplification testing requires respiratory samples from the patients and nasopharyngeal swabs are most commonly used. Molecular tests can yield a false negative result if the level of viral RNA in a particular sample is too low for detection, and results can be skewed if the samples were not properly taken. According to the clinical studies, the sensitivity of the molecular test is only around 50%. [1]
As new tools, like serological testing, become readily available, we must have a better understanding of different COVID-19 testing, so that we can best use the existing tools to effectively control the spread of SARS-CoV-2.
The immune system naturally produces antibodies in response to infection. Serological tests are diagnostic methods used to identify antibodies and antigens in a patient's sample. Compared to the molecular test, the serological test usually has the advantages of low detection threshold, convenient and stable sample collection, high throughput, and low workload. To characterize the prevalence and spread of the disease, serological testing is widely carried out, and the demand for SARS-CoV-2 serological testing is rapidly increasing.
So far, 12 have got emergency use authorization from FDA (Table 1), and 16 serological tests have been approved by the CFDA for emergency use (Table 2), and the assay formats include colloidal gold immunoassay, chemiluminescence immunoassay, and ELISA.
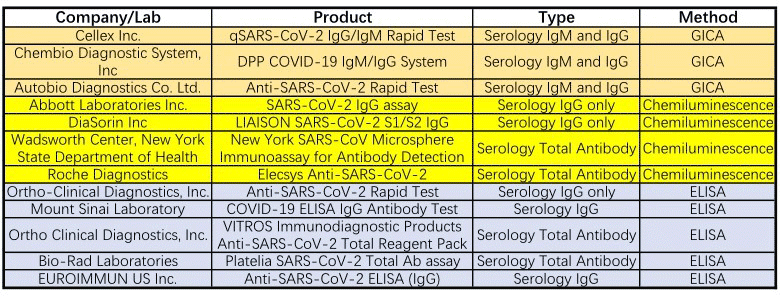
Table 1 Emergency Use Authorizations for COVID-19 by FDA
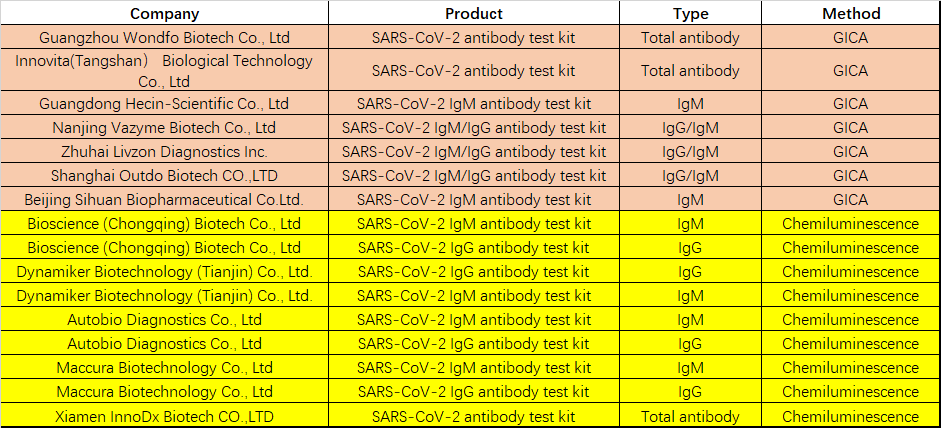
Table 2 COVID-19 diagnostic approved by CFDA
We have discussed the mechanism and performance of those three formats in our previous articles. Below is a summary to refresh your mind. (Table 3, Fig.1, Fig.2 and Fig.3)
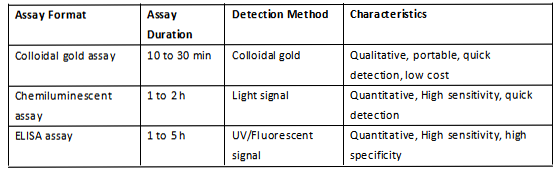
Table 3 Comparison of three assay formats.
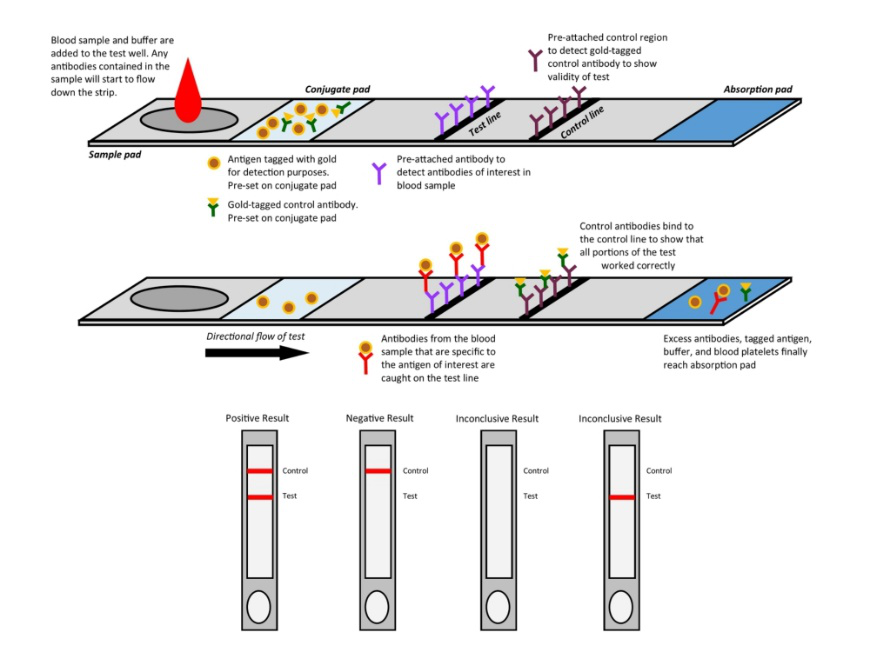
Fig. 1 Mechanism of colloidal gold assay
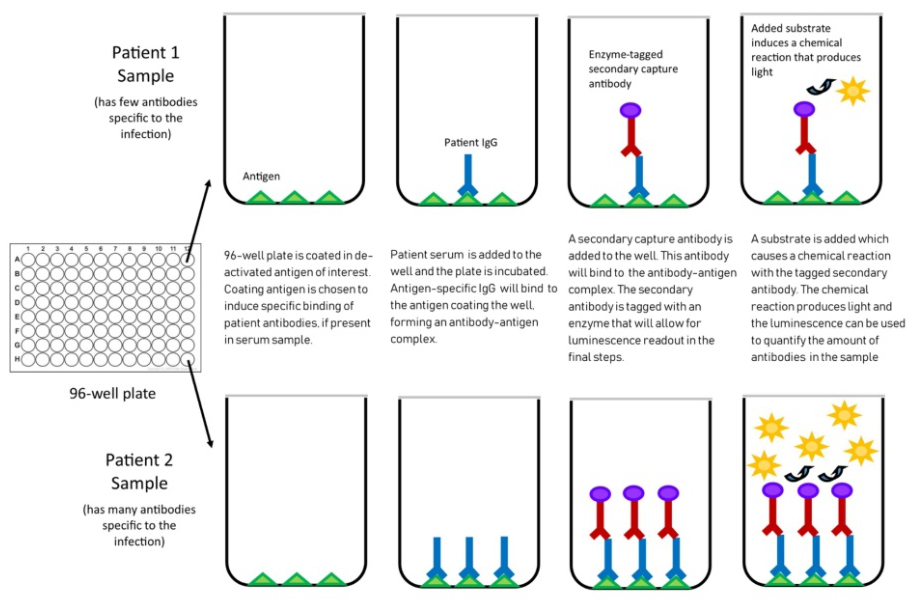
Fig. 2 Mechanism of chemiluminescent assay
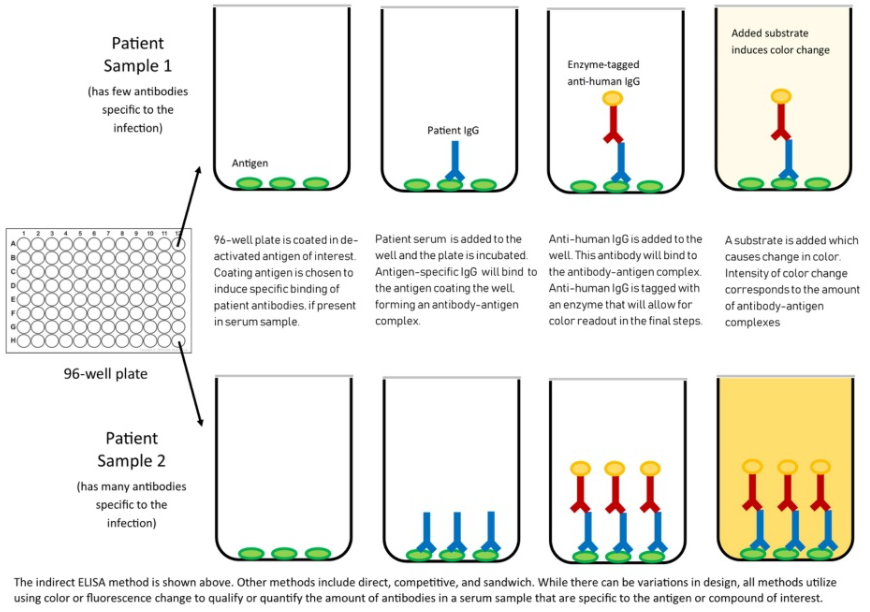
Fig. 3 Mechanism of ELISA
(Source: https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html)
In common, all three methods above use antigens to capture the target antibody. However, the accuracy of the test is significantly affected by the antigen reagents. To better support the development of serological test kits, ACROBiosystems has specifically designed and optimized SARS-CoV-2 antigen products for serological test kits, including S1, N, wild type RBD as well as mutant RBD proteins with various tags.
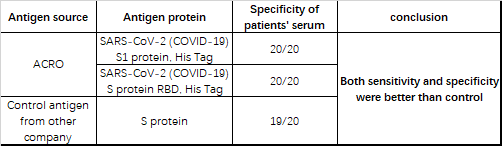
Our products are verified by a lot of customers using their serological assay platforms and we received a lot of positive feedbacks (Table 4). Recently, we got the good news that one of our customers successfully launched their chemiluminescent COVID-19 antibody detection kit using ACRO's biotin-labeled S1 protein. The sensitivity of this kit reached 93.2% and the specificity was 100%.
Application in GICA
Application in ELISA
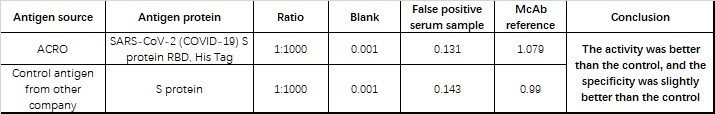
Table 4 Feedback data using ACRO’s SARS-CoV-2 reagents
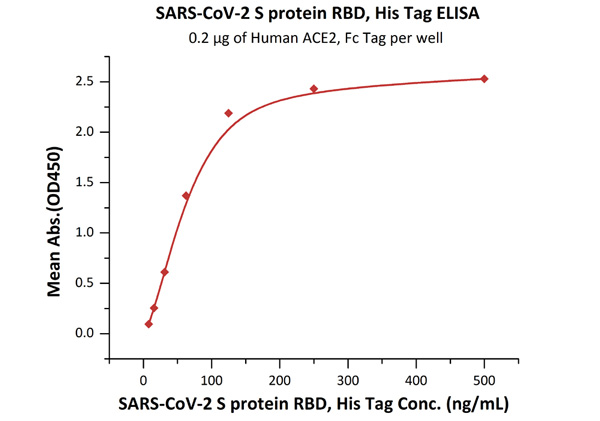
What’s more, ACROBiosystems has developed the Detection of Anti-SARS-CoV-2 antibody assay kit (Spike protein RBD), which uses an indirect ELISA method. We coat SARS-CoV-2 S protein RBD, His Tag (Cat.No. SPD-C52H3) to detect anti-SARS-CoV-2 IgG antibody in serum.
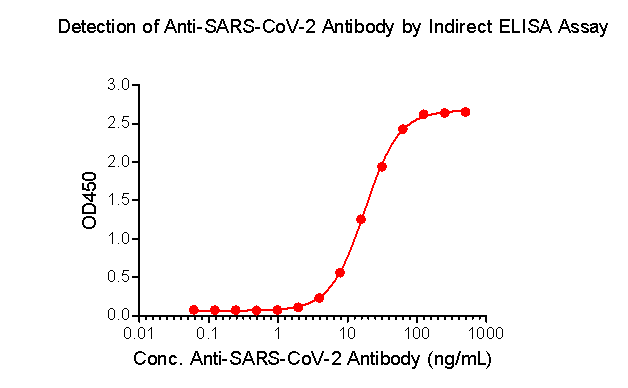
Fig 4. Indirect ELISA method to detect anti-SARS-CoV-2 IgG antibody
ACRO’s verified protein reagents can meet your custom requirements and supply in large quantities. Click here to submit your request.
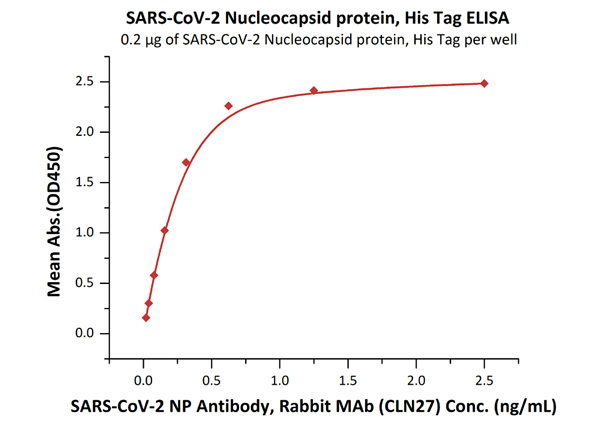
Fig.1 Immobilized SARS-CoV-2 Nucleocapsid protein, His Tag (Cat. No. NUN-C51H9) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) with a linear range of 0.02-0.3 ng/mL.
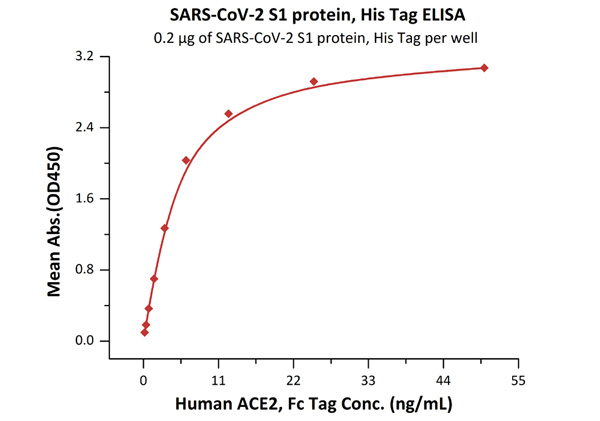
Fig.2 Immobilized SARS-CoV-2 S1 protein, His Tag (Cat. No. S1N-C52H4) at 2 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.2-6 ng/mL.
>>> Check all SARS-CoV-2 proteins and inhibitory kits.
Reference:
1. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019
This web search service is supported by Google Inc.