
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Identification and characterization of neutralizing antibodies against COVID-19 Just weeks after parts of the U.S. began reopening, the COVID-19 infections are on the upswing in several states. As the COVID-19 pandemic continues, researchers and manufacturers are moving potential therapeutics into clinical trials at a dizzying pace. During the past two weeks, Eli Lilly and Regeneron successively announced their clinical trials of therapeutic antibody against COVID-19.
The development cycle of therapeutic antibodies is relatively short. Thus, many experts believe the efforts of the companies developing antibody drugs are so vital. Compared to vaccines or antiviral drugs, the therapeutic antibody’s potential to both treat and protect against viral infections makes them very unique. With the advantage of high specificity and easy to scale up, the neutralizing antibody development is drawing as much attention around the world.
Since the very beginning of the pandemic, ACROBiosystems has been committed to developing SARS-CoV-2 related protein reagents and kit products to support the development of diagnostics, vaccines, and therapeutics. Meanwhile, ACROBiosystems is also actively involved in the identification and characterization of neutralizing antibodies against COVID-19. Collaborating with East China Normal University, the First Affiliated Hospital of Zhejiang University, School of Medicine, and SymRay Biopharma Inc., ACROBiosystems recently published an article “Cross-neutralization antibodies against SARS-CoV-2 and RBD mutations from convalescent patient antibody libraries”.
1. The binding affinity of plasma to SARS-CoV-2 RBD
We examined the binding ability of plasma from 18 recovered COVID-19 patients to SARS-CoV-2 RBD by enzyme-linked immunosorbent assay (ELISA). We found that most of these convalescent patients were able to produce high titer of SARS-CoV-2 RBD-specific antibodies when compared with healthy donors (Fig. 1).
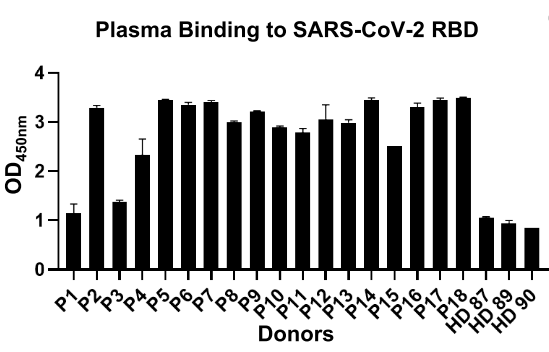
Fig. 1 The binding affinity of plasma from each patient to RBD by ELISA. (P1-18, Patients with COVID-19 recovered. HD87-90, healthy donors)
2. Generation of patient-derived antibody library
The PBMC from 18 different COVID-19 recovered patients were isolated to generate phage-displayed scFv libraries for panning the neutralizing antibodies. Affinity selection of the patient-derived antibody library was performed using solid-phase-bound RBD. After three rounds of panning, 456 positive clones were identified to bind with RBD specifically. 19 of them were selectively expressed for the following steps.
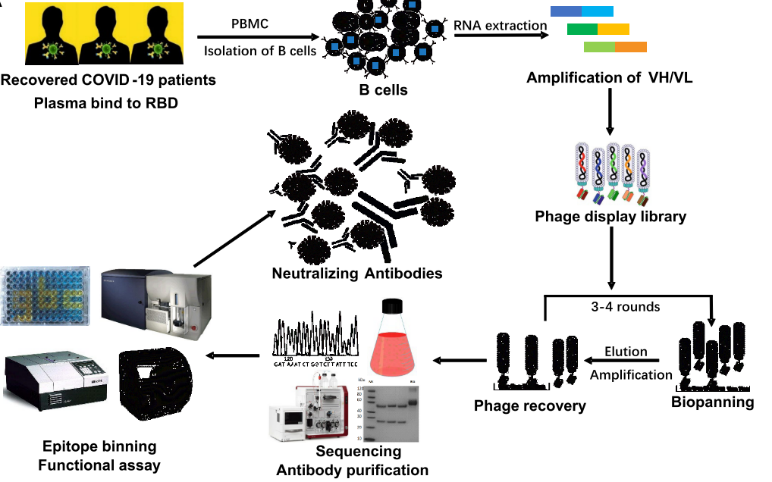
Fig. 2 The flow chart of construction and panning of patient-derived scFv libraries.
3. Epitope binning assay
19 IgG can be divided into six different Bins using the Octet method. Among them, Bin1 and Bin2 are the groups with most clones, suggesting that these two epitopes on RBD are most important for recognition by the humoral immune system.
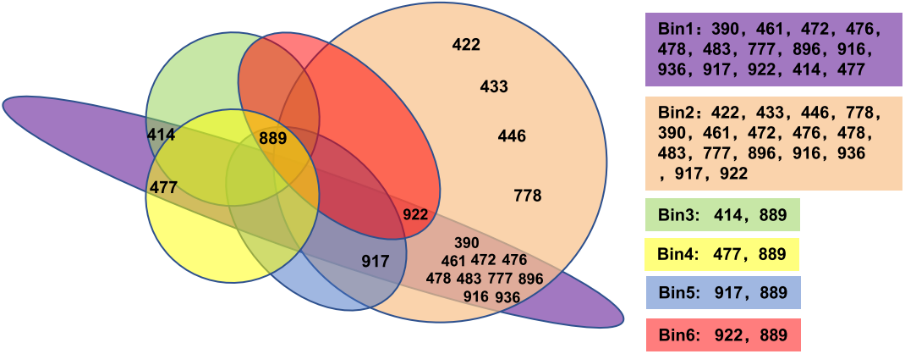
Fig. 3 The 19 antibodies were divided into 6 bins by epitope binning assay.
4. Cross-neutralization against SARS-CoV-2 RBD mutations
According to the in vitro binding assay, some IgG in Bin2 show superior bioactivity in the blockade of RBD binding to human ACE2 cells. We chose four antibodies from Bin2 to further characterize the neutralizing activity against SARS-CoV-2 RBD mutants. As you can see from the Fig. 4, HTS0483 can bind with all the RBD mutants and successfully inhibit the binding between ACE2 and all RBD mutants, indicating that HTS0483 can be potentially developed into the therapeutics for COVID-19.
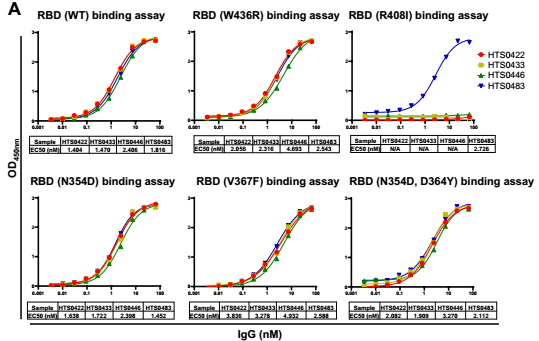
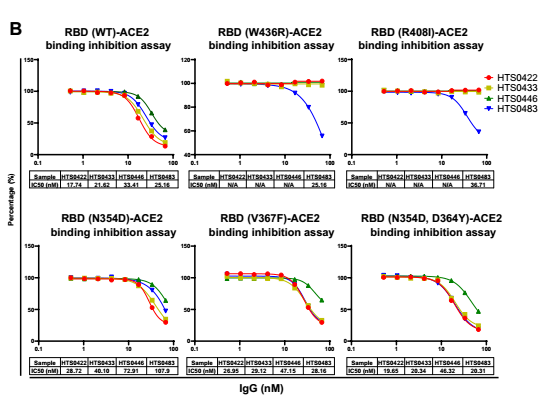
Fig. 4 Cross-neutralization against SARS-CoV-2 RBD mutations by different antibodies. (A) The binding affinity of 4 positive antibodies from Bin2 to different SARS-CoV-2 RBD mutations. (B) The blocking ability of 4 positive antibodies from Bin2 to different SARS-CoV-2 RBD mutations
5. Antiviral properties of human antibodies
All four antibodies from Bin2 showed very good neutralizing activities in the antiviral studies. The IC50 is between 12.5nM to 50nM.
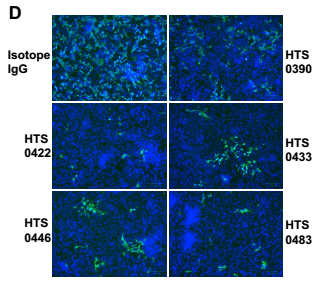
Fig. 5 Antiviral properties of human IgG antibodies. The authentic infection of SARS-CoV-2 to Vero-E6 cells was neutralized by Anti-RBD antibodies.
ACROBiosystems has developed anti-SARS-CoV-2 S protein RBD neutralizing antibody and Nucleocapsid antibody. In addition, kit products for neutralizing antibody screening and antibody titer assay, and recombination proteins are provided to accelerate the anti-SARS-CoV-2 drugs and vaccines R&D.
RBD and N antibody
——Human antibodies isolated from the serum of recovered patients
> As a control to develop various assays
> Accelerating SARS-COV-2 diagnostic kits, therapeutics and vaccines R&D
——Multiple antibody titer assay kits
> High throughput, high flexibility, high reproducibility, high sensitivity and high stability
> Various assay kits available for different application of antibody titer measurement
——Inhibitor screening kit/pre-coupled magnetic beads/pre-coated plates
> High throughput inhibitor screening kit can screen 96 or 480 candidate antibodies simultaneously
> Ready-to-use beads and plates can improve the efficiency of neutralizing antibody screening
Relevant proteins
Reference:
1. Cross-neutralization antibodies against SARS-CoV-2 and RBD mutations from convalescent patient antibody libraries. doi: https://doi.org/10.1101/2020.06.06.137513
This web search service is supported by Google Inc.







