Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Biosimilar Drug Targets
Biosimilars are biologic drugs that are "highly similar" to a previously approved biologic (also known as reference innovator product). A biosimilar must undergo a stringent similarity testing at every step of its development to demonstrate that potential differences from the reference product are not clinically meaningful with regard to quality, safety, and efficacy [EMA], or safety, purity, and potency [FDA].
The binding between a biosimilar and its target antigen (and Fc receptors) is considered an important indicator to its efficacy. ACROBiosystems has developed high quality target antigen proteins and Fc receptor proteins to facilitate biosimilar research.
Most target antigens and Fc receptors offered by ACROBiosystems have been validated with the reference innovator drugs in functional ELISA, BLI or SPR. The reference innovator drugs include Herc*ptin®, Avastin®, MabThera®, Erbitux® and Humira®, which are purchased from Roche, Merck KGaA, and AbbVie, respectively.
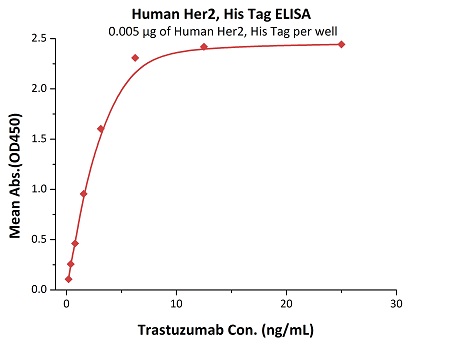
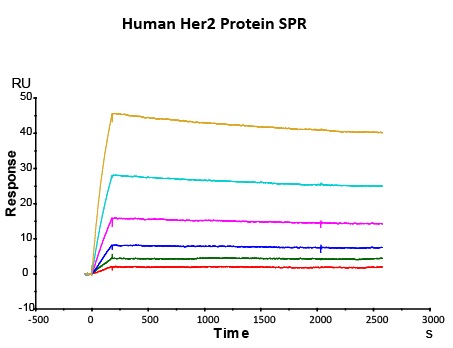
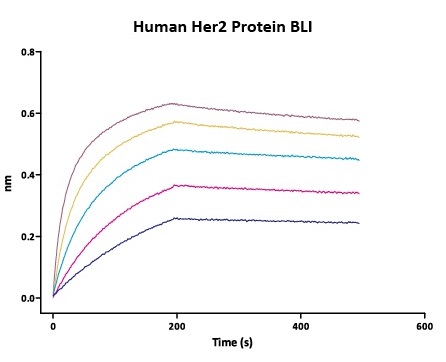
We routinely apply rigorous quality control measures to ensure consistent performance of our product. Newly produced products are subjected to side-by-side comparison with our internal standard in a variety of assays. Only those within an acceptable margin of difference are allowed to be released.
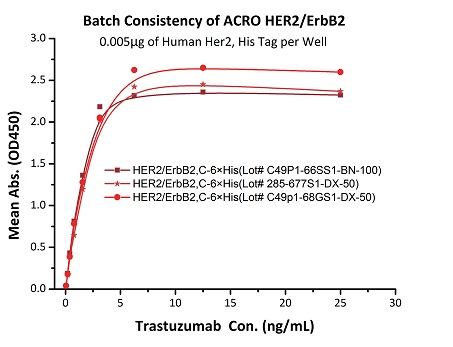
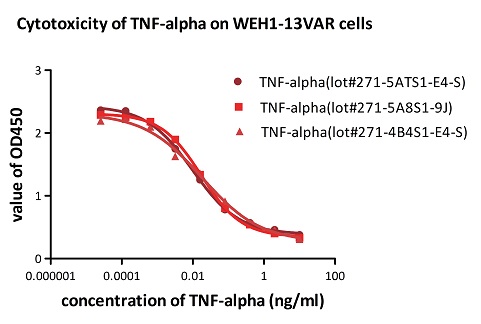
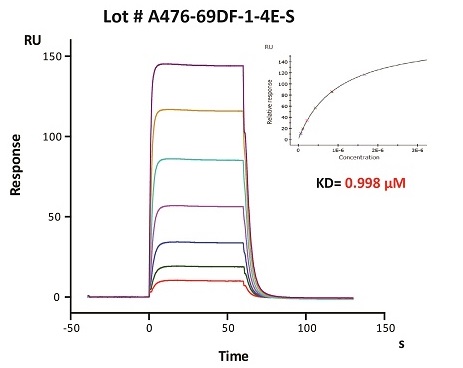
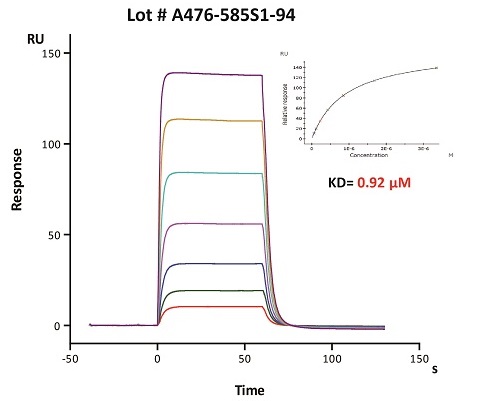
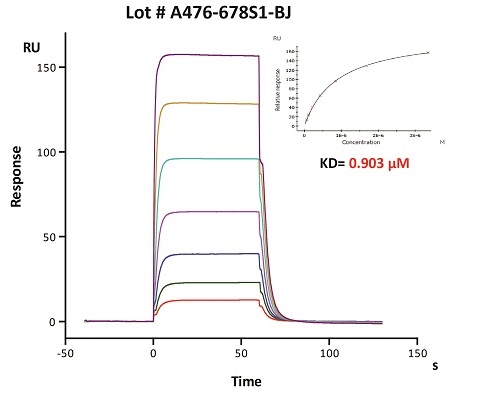
To ensure the stability of proteins, we do the stability testing through accelerated and long-term stability testing. After testing, protein retains its quality and no activity loss is observed over the life span of the product.
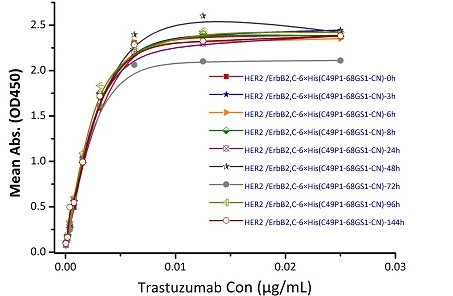
Stability of Her2 under 37℃ was Determined by SDS-PAGE and ELISA
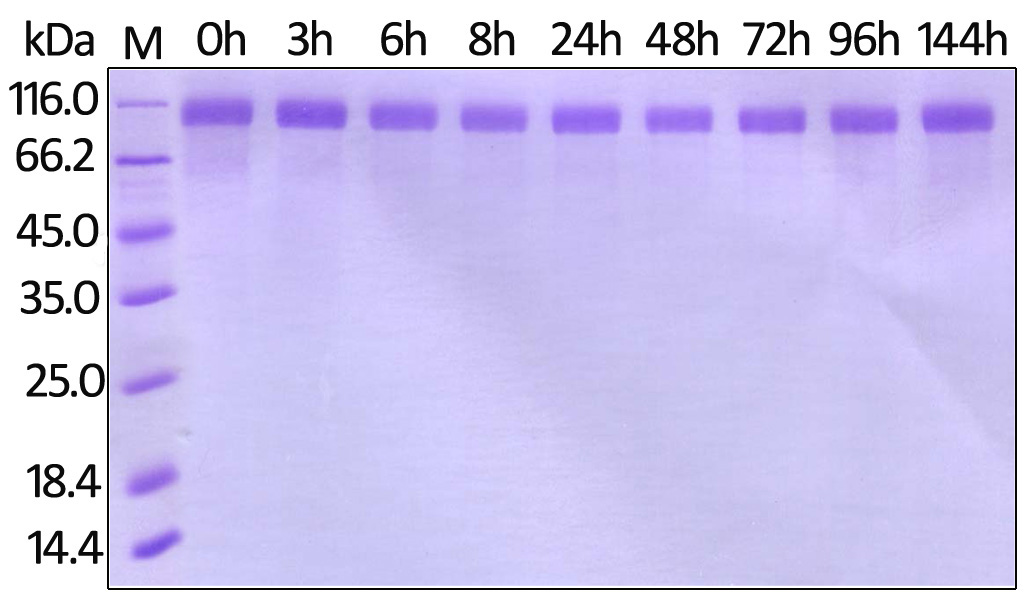
To meet the high purity requirement of pharmaceutical applications, most of our proteins have to go through both SDS-PAGE and HPLC analyses. Only those meeting all requirements will be issued a lot-specific certificate of assurance and be released.


Charge variant analysis of proposed biosimilar to Trastuz*mab
Authors: Dakshinamurthy P, et al.
Journal: Biologicals 2017
Application: SPR
Product: HE2-H5225
HER2-positive breast cancer targeting and treatment by a peptide-conjugated mini nanodrug
Authors: Ding H, et al.
Journal: Nanomedicine 2016
Application: SPR
Product:HE2-H5253
Development and validation of a cell-based fluorescent method for measuring antibody affinity
Authors: Yu X, et al.
Journal: J Immunol Methods 2016
Application: Cell-based assay
Product:EGR-H5222
Authors: El Amrani M, et al.
Journal: J Chromatogr A 2016
Application: ELISA
Product: TNA-H8211
Monoclonal antibodies against HER2 epitope and methods of use thereof
Authors: Natalya D Bodyak, et al.
Journal: US20150366987A1 2015
Application: ELISA
Product: HE2-H5225
Generation of a canine anti-EGFR (ErbB-1) antibody for passive immunotherapy in dog cancer patients
Authors: Singer J, et al.
Journal: Mol Cancer Ther 2014
Application: ELISA
Product:EGR-H5222
In vitro Fab display: a cell-free system for IgG discovery
Authors: Stafford RL, et al.
Journal: Protein Eng Des Sel 2014
Application: SPR(Biacore T200)
Product:VE1-H4213
This web search service is supported by Google Inc.