
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
SSTR2 (Somatostatin Receptor2) was originally discovered by radioligand binding studies in pituitary tumor cell lines, in which somatostatin (SST) can effectively inhibit the secretion of prolactin and GH (growth hormone). Subsequent radioligand binding experiments showed that SSTR2 is widely distributed in many tissues.
Five different SSTR genes have been found, named SSTR1-SSTR5, of which SSTR2 is the most studied.
SSTR2 mRNA and protein are widely expressed in normal tissues, but have low expression consistency. The protein form of SSTR2 has the highest expression in the brain and kidneys. At the same time, SSTR2 is widely expressed in solid tumors. Since it is highly expressed in neuroendocrine tumors (NETs), SSTR2 is the key target for the treatment of NETs at present.
Recent studies have shown that SSTR2 is significantly methylated in colorectal cancer and has also been implicated in tumorigenesis in gastric and breast cancers. Furthermore, the researchers found that SST binding to SSTR2 inhibited the release of cytokines from immune cells and impacted on the tumor microenvironment (TME).
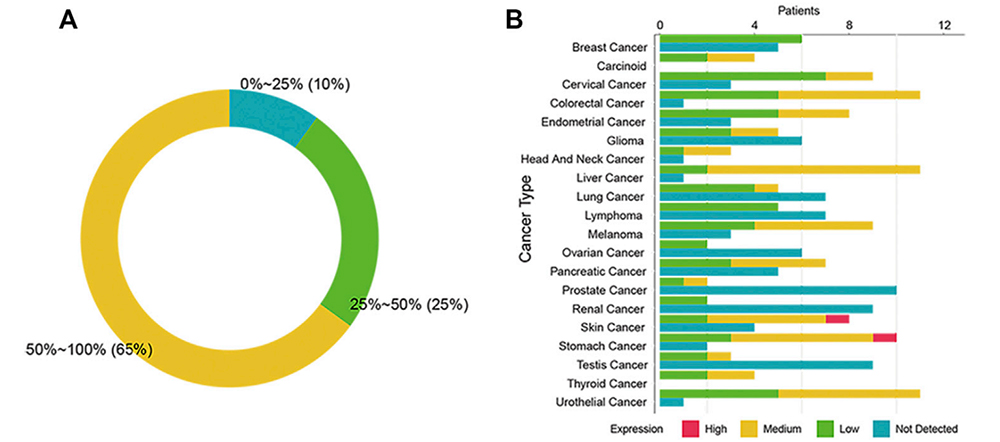
The Expression of SSTR2 in 20 cancers from the Human Protein Atlas
To study the expression of SSTR2 in different cancers, the team from Xinqiao Hospital of Third Military Medical University integrated IHC data from the Human Protein Atlas. The results showed that the IHC positive rate of SSTR2 in 13 cancers was higher than 50%; 5 cancers slightly expressed SSTR2 (positive rate 25% ~ 50%); 2 cancers slightly expressed SSTR2 (positive rate 0% ~ 24%). It can be seen that the expression of SSTR2 is differential and widely distributed in various cancer types. Furthermore, cell-mediated immunity may be better activated and maintained in patients with high SSTR2 expression.
SSTR2 is a member of the G protein-coupled receptor (GPCR) family and is a seven-pass transmembrane protein with the N-terminal extracellular and the C-terminal intracellular. Studies have shown that some SSTRs can heterodimerize with other members of the SST family and unrelated GPCRs, including DRD2, β-adrenergic receptors. The co-expression of such interacting receptors in cells can affect SSTRs transport, signal transduction, or biological activity. Therefore, the role of these heterodimers in physiological or pathological conditions will be an interesting area for future research.
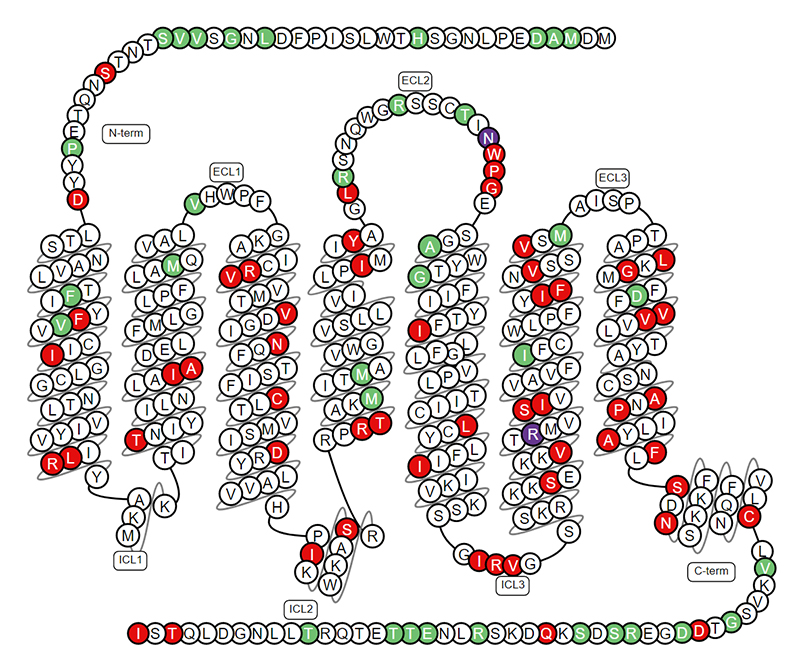
The Structure of SSTR2
SSTR2 inhibits the release of many hormones and other secreted proteins by binding to the SST, inhibiting cell proliferation and angiogenesis. The antitumor activity of SST occurs through direct and indirect mechanisms:
(a) Binding to SSTR on the tumor surface activates downstream cell cycle arrest or related apoptotic factors.
(b) SSTR induces tumor angiogenesis and the production of tumor growth inhibitors. SSTR2 gene expression is absent in 90% of human pancreatic cancers. The construction of human pancreatic cells expressing SSTR2 (without expressing endogenous SSTR2) induces the expression of its ligand (SST), thereby inhibiting cell proliferation, tumorigenicity, and metastasis.
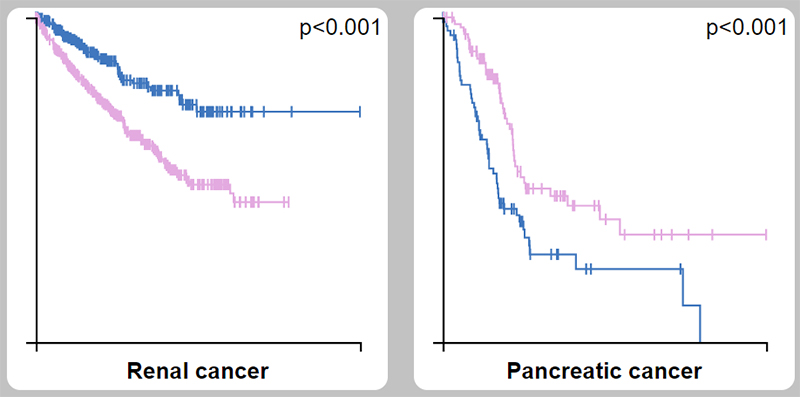
SSTR2 as a prognostic marker in kidney cancer (unfavorable) and pancreatic cancer (favorable)
Tidutamab (XmAb-18087) is a representative drug targeting SSTR2 which is developed by Xencor. As a bispecific antibody (IgG1), XmAb-18087 can bind to CD3 to activate T cells for the efficient and targeted killing of SSTR2-expressing tumor cells. XmAb-18087 is currently in Phase I/II, and its main indications are neuroendocrine tumors and lung cancer.
ACROBiosystems has successfully developed HEK293 expressed full-length SSTR2-VLP protein (Met 1-Ile 369) via "FLAG" to support drugs and therapies R&D targeting SSTR2.
| Molecule | Cat. No. | Species | Host | Product Description | Application |
|---|---|---|---|---|---|
| SSTR2 | SS2-H5216 | Human | HEK293 | Human SSTR2 Full Length Protein-VLP (HEK293) | Immunization ELISA SPR/BLI Cell-based Assay … |
| VLP | VLP-N5213 | Virus-Like Particle (VLP) isotype control VLP | Isotype Control | ||
![]() Full-length SSTR2 protein with native and complete conformation
Full-length SSTR2 protein with native and complete conformation
![]() Higher immunogenicity due to inherent characteristics of VLP
Higher immunogenicity due to inherent characteristics of VLP
![]() High biological activity is verified by binding to antibodies
High biological activity is verified by binding to antibodies
![]() 100-300 nm in size, can be used as an optimal target for dendritic cells and phage display
100-300 nm in size, can be used as an optimal target for dendritic cells and phage display
![]() Suitable for immunization/ELISA/SPR/BLI/cell-based assay, etc.
Suitable for immunization/ELISA/SPR/BLI/cell-based assay, etc.
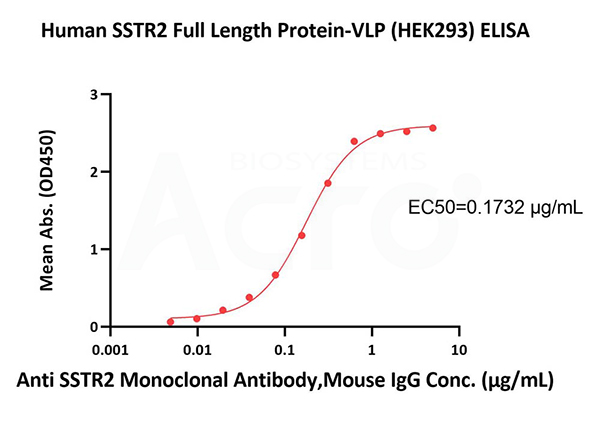
Immobilized Human SSTR2 Full Length Protein-VLP (Cat. No. SS2-H5216) at 10 μg/mL (100 μL/well) can bind Anti SSTR2 Monoclonal Antibody, Mouse IgG with a linear range of 0.005-0.625 μg/mL (QC tested).
>>> Learn more about “FLAG”: full-length multi-pass transmembrane proteins and technology platforms
1.Marily Theodoropoulou, Günter K. Stalla. Somatostatin receptors: From signaling to clinical practice. Frontiers in Neuroendocrinology (2013). http://dx.doi.org/10.1016/j.yfrne.2013.07.005
2.Aoyun Wang, Yixiao Yuan, Han Chu, et al. Somatostatin Receptor 2: A Potential Predictive Biomarker for Immune Checkpoint Inhibitor Treatment. Pathol. Oncol. (2022) https://doi.org/10.3389/pore.2022.1610196
This web search service is supported by Google Inc.







