 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
The evolution of the immune system follows two basic rules: "destroy foreign objects when in danger, "Don't seriously harm the host". It is a difficult task to selectively treat normal cells and avoid immune lesions. Therefore, inflammation stimulates the regulation mechanism and starts tissue repair to restore physiological functions and internal stability. Interferon plays a core role between immune cells and non -immune cells.
IFN-γ is the only member of the type II interferon family and is a soluble cytokine. From a biological perspective, IFN-γ is a multi-effect cytokine with antiviral, antitumor and immune regulation. Therefore, IFN-γ plays an important role in coordinating congenital and adaptive immune reactions.
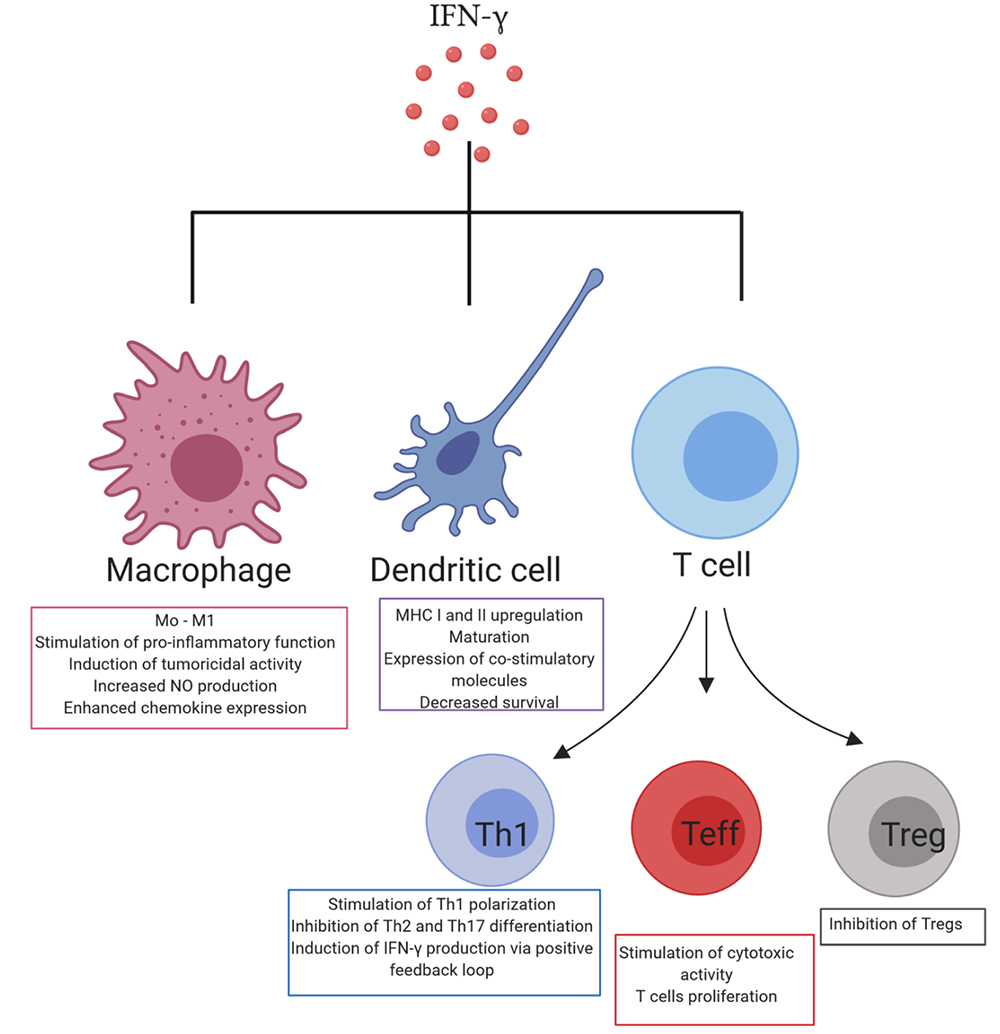
The interaction of IFN-γ with immune cells
In an inflammatory environment, IFN-γ triggers the activation of an immune response that stimulates the clearance of pathogens, and it also prevents overactivation of the immune system and tissue damage, although the complex mechanisms that maintain this balance are not fully understood. In the tumor microenvironment (TME), IFN-γ always plays a coordinated anti-tumor and pro-tumor immune function. IFN-γ, together with granzyme B and perforin, act as cytotoxic cytokines to initiate tumor cell apoptosis. IFN-γ can also synthesize immune checkpoint inhibitory molecules and indoleamine 2,3-dioxygenase (IDO), thereby stimulating other immune suppression. Thus, although IFN-γ is a key factor in antitumor immunity, it can initiate an alternative program in tumor cells, providing tumor cells with additional capacity to respond to immune responses.
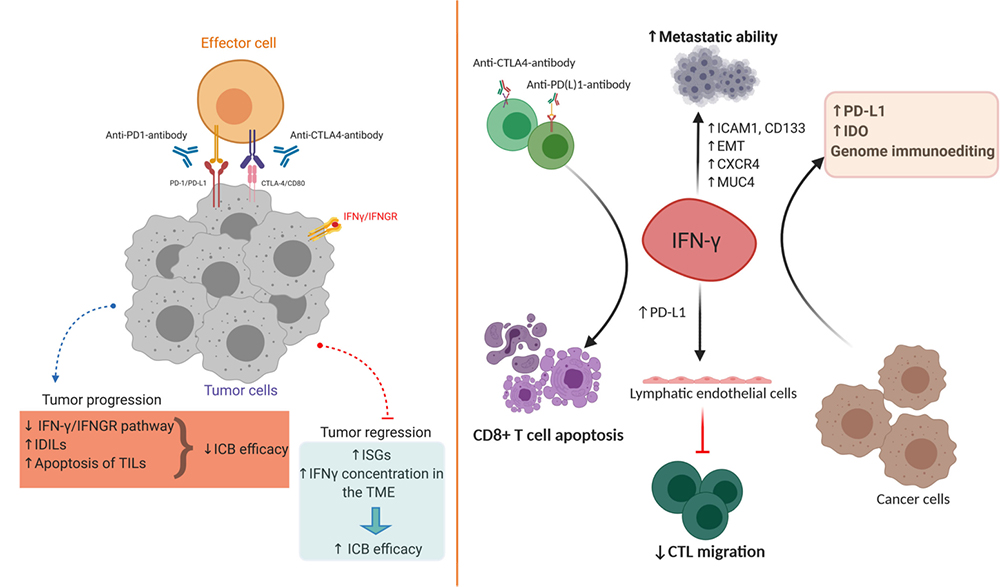
Pro-tumor and anti-tumor effects of IFN-γ
IFN-γ is a protein encoded by the IFNG gene, consisting of two antiparallel polypeptide chains. Fully synthetic IFN-γ is glycosylated at the amino terminus and the level of glycosylation determines the final weight of the defined moiety. It has been reported that glycosylation itself does not affect the activity of interferon, but prevents its degradation by proteases. Thus, this chemical modification increases the half-life of IFN-γ in blood, prolonging its mediated effects.
ACROBiosystems developed recombinant IFN-γ proteins (Cat. No. IFG-H4211) expressed by HEK293 to achieve the post-translational glycosylation modification and correct protein folding; the high biological activity is verified by binding its receptor IFN-γ R1 and cell-based experiments. Click the form below to view more information and verification data of IFN-γ and IFN-γ R1 proteins.
Product list:IFN-γ and IFN-γ R1
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
| IFN-γ | IFG-H4211 | Human | ActiveMax® Human IFN-gamma / IFNG Protein, Tag Free |
| IFN-γ R1 | IF1-H5223 | Human | Human IFN-gamma R1 /IFNGR1 Protein, His Tag |
| IFN-γ R1 | IF1-H5254 | Human | Human IFNGR1 / CD119 Protein, With C-Fc Tag |
FN-γ-mediated host-pathogen interactions are critical for studying disease mechanisms and also exhibit enormous therapeutic importance for the elimination of various infections and autoimmune diseases. T cells capable of recognizing and eliminating cancer cells were identified based on IFN-γ positivity during CAR-T cell therapy. In addition, selection of effector T cell clones is generally based on their IFN-γ production capacity. However, the expression of IFN-γ and the efficiency of CAR-T cell therapy in vivo are not always positively correlated, which suggesting an alternative role for IFN-γ in cancer immunoediting. Nonetheless, IFN-γ is still considered to be one of the most valuable parameters for predicting clinical response.
The production of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) by CAR-T cells can enhance antigen presentation, cell adhesion, and recruitment of Th1 cells, thereby enhancing host antitumor immunity. In addition, in order to improve the success rate of CAR-T cell therapy or prevent tumor recurrence, tumor-infiltrating macrophages need to be regulated by IFN-γ.
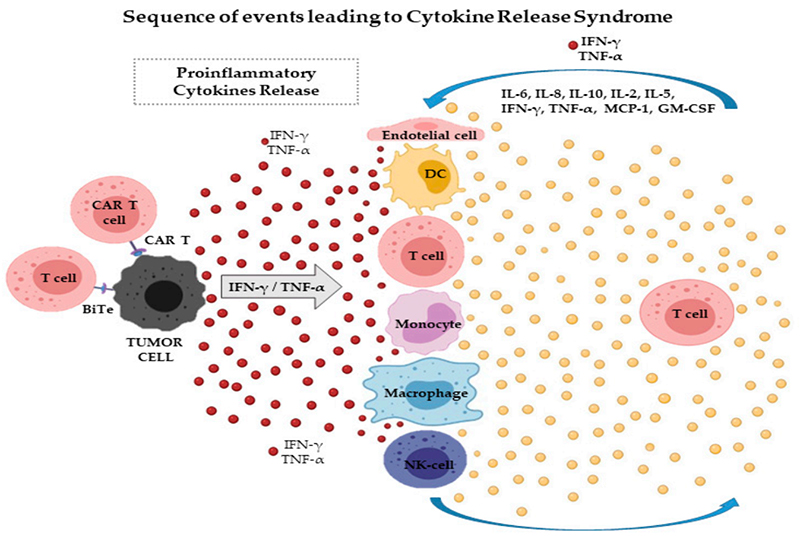
CAR-T cells target tumor cells and induce the release of cytokines such as IFN-γ
Cytokine release syndrome (CRS) and macrophage activation syndrome (MAS) are serious immunopathological consequences of adoptive T cell therapy including CAR-T. IFN-γ is thought to be a common player in these syndromes. In addition to increased levels of IFN-γ, mediators such as IL-6, IL-10, IFN-γ-induced chemokines, and TNF-α also contribute to potentially lethal toxicity and other adverse effects. Especially when chronically exposed to IFN-γ, macrophages differentiate into hemophagocytic cells and exacerbate the disease, suggesting that MAS has an IFN-γ-dependent physiopathology.
Therefore, the detection and monitoring of IFN-γ is particularly important in clinical practice.
At present, the detection of IFN-γ is usually carried out by the method of antigen-antibody specific binding. ACROBiosystems has launched three human-mouse chimeric anti-IFN γ antibodies derived from different clones: they can specifically bind to Human IFN-γ; they have been verified to be suitable for the detection of IFN-γ by antibodies sandwich method.
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
| Anti-IFNγ antibody | IFN-M411 | Human | Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H4) |
| IFN-M412 | Human | Monoclonal Anti-IFNγ antibody, Human IgG1 (8C5F8) | |
| IFN-M414 | Human | Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H6) |
![]() High purity: greater than 95% as verified by SDS-PAGE
High purity: greater than 95% as verified by SDS-PAGE
![]() High specificity: only specifically binds to IFN-γ, not to other cytokines (IL-2, IL-4, IL-6, IL-10, GM-CSF, TNF-alpha)
High specificity: only specifically binds to IFN-γ, not to other cytokines (IL-2, IL-4, IL-6, IL-10, GM-CSF, TNF-alpha)
![]() High biological activity: the binding activity and affinity to IFN-γ are verified by ELISA, SPR and BLI
High biological activity: the binding activity and affinity to IFN-γ are verified by ELISA, SPR and BLI
![]() High sensitivity: suitable for the detection of IFN-γ and the development of detection methods, the sensitivity can reach pg grade
High sensitivity: suitable for the detection of IFN-γ and the development of detection methods, the sensitivity can reach pg grade
Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H4) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.

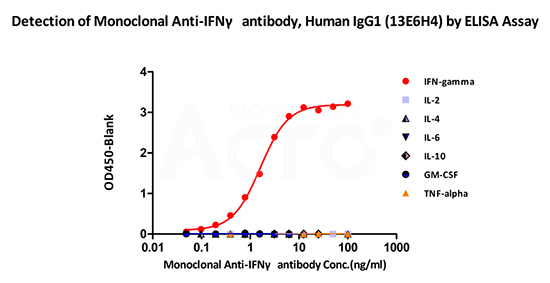
Immobilized ActiveMax® Human IFN-gamma, Tag Free (Cat. No. IFG-H4211) can bind Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H4) (Cat. No. IFN-M411) with a linear range of 0.09-1.56 ng/mL (QC tested). No cross-reactivity is detected with other human cytokines, including IL-2, IL-4, IL-6, IL-10, GM-CSF and TNF-alpha.
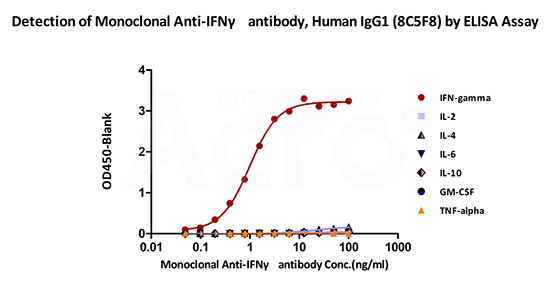
Monoclonal Anti-IFNγ antibody, Human IgG1 (8C5F8) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.

Immobilized ActiveMax® Human IFN-gamma, Tag Free (Cat. No. IFG-H4211) can bind Monoclonal Anti-IFNγ antibody, Human IgG1 (8C5F8) (Cat. No. IFN-M412) with a linear range of 0.09-1.56 ng/mL (QC tested). No cross-reactivity is detected with other human cytokines, including IL-2, IL-4, IL-6, IL-10, GM-CSF and TNF-alpha.
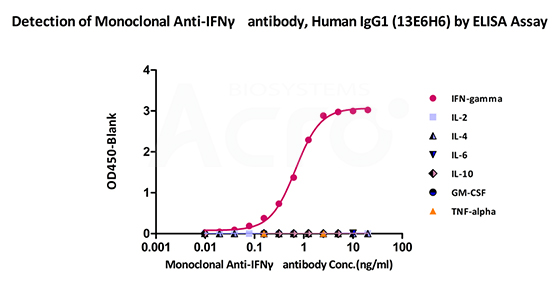
Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H6) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.

Immobilized ActiveMax® Human IFN-gamma, Tag Free (Cat. No. IFG-H4211) can bind Monoclonal Anti-IFNγ antibody, Human IgG1 (13E6H6) (Cat. No. IFN-M414) with a linear range of 0.07-0.63 ng/mL (QC tested). No cross-reactivity is detected with other human cytokines, including IL-2, IL-4, IL-6, IL-10, GM-CSF and TNF-alpha.
We will launch other members of ACRO "Antibody Matrix" in succession. If you have any needs or more development suggestions, don't hesitate to contact us.
1. M. Alper Kursunel, Gunes Esendagli. The untold story of IFN-γ in cancer biology, Cytokine & Growth Factor Reviews (2016). https://doi.org/10.1016/j.cytogfr.2016.07.005.
2. Jorgovanovic, D., Song, M., Wang, L. et al. Roles of IFN-γ in tumor progression and regression: a review. Biomarker Research (2020). https://doi.org/10.1186/s40364-020-00228-x
3. Maria Cosenza, Stefano Sacchi, Samantha Pozzi. Cytokine Release Syndrome Associated with T-Cell-Based Therapies for Hematological Malignancies: Pathophysiology, Clinical Presentation, and Treatment. J. Mol. Sci. (2021). https://doi.org/10.3390/ijms22147652
This web search service is supported by Google Inc.
