
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Allogeneic CAR T-cell therapy, as known as “off-the-shelf” CAR-T. The manufacturing process of allogeneic CAR-T cell products including apheresis, gene editing, amplification, harvest, storage and other steps (Fig 1).
1) Apheresis: Isolate T lymphocytes cell from a healthy donor’s blood.
2) T-cell manufacturing:Transfect CAR and other genes into T lymphocytes cells through viral vector-mediated transgene or gene editing technology. In addition, it’s also crucial to prevent the expression of a functional TCR on the surface of αβ T cells via gene editing technology for reducing the GvHD risk.
3) Amplification:Anti-CD3/anti-CD28 beads and cytokines are used to expand T cells.
4) Harvest and storage: Allogeneic CAR-T cells are harvest, cryo-preserved and stored in a secure cell bank.
5) Infusion: T cells are infused into new patients, on-demand.
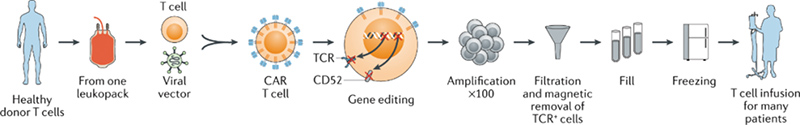
Fig. 1 Manufacturing of allogeneic CAR T cells
There are a number of advantages to using allogeneic CAR-T cells including reduced cost of goods, a more simplified supply chain and better characterization/ quality testing of the start and end products, which are common issues associated with autologous CAR-T cells. (Fig 2)
1) It can be "off-the-shelf " supply without long complicated process.
2) T lymphocytes cells come from healthy donors with strong activity, and the T cells can effectively avoid tumor contamination after strict screening procedures.
3) It is also possible to generate many therapeutic doses from a single manufacturing lot that could be readily available to treat multiple patients. This drives down the cost of treatment per patient substantially compared to autologous therapies.
4) The quality of production process can be controlled with GMP standard.
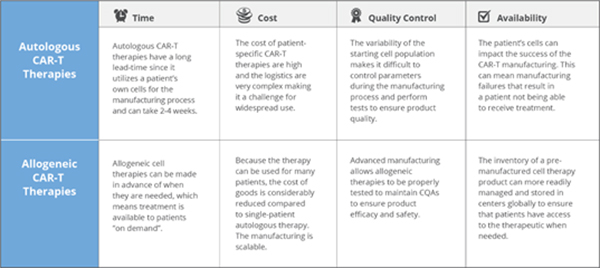
Fig.2 Comparison between autologous and allogeneic CAR T cells
How many companies are conducting allogeneic CAR-T (UCAR-T) cell therapy pipelines?
ACROBiosystems has successfully developed an extensive collection of recombinant proteins, such as BCMA, CD19, and MSLN, for allogeneic CAR-T therapy development. This growing list of proteins includes many fluorescent-labeled target antigens , biotinylated proteins and unconjugated proteins that are uniquely suitable for evaluation of CAR expression.
FACS Data
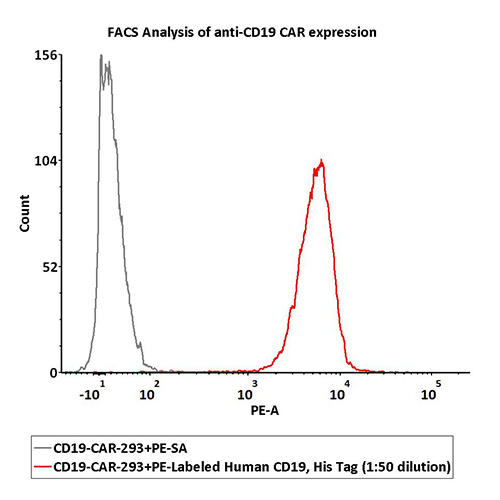
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as negative control
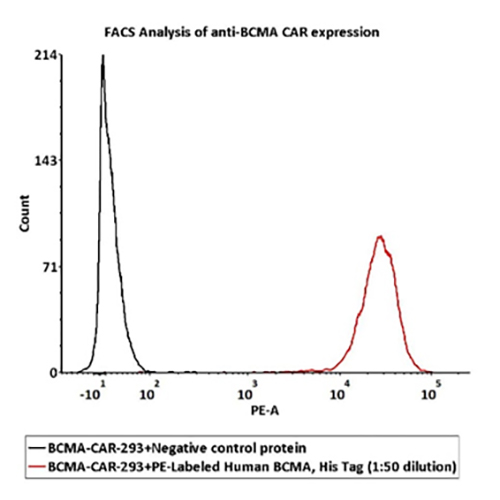
1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No. BCA-HP2H2) and negative control protein respectively, PE signal was used to evaluate the binding activity.
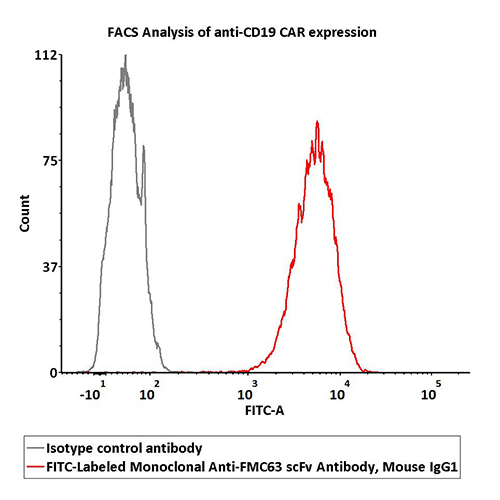
2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was used to evaluate the binding activity.
Reference
[1] Depil, S et al. “'Off-the-shelf' allogeneic CAR T cells: development and challenges.” Nature reviews. Drug discovery vol. 19,3 (2020): 185-199.
[2] https://www.allcells.com/car-t-cell-therapy/
This web search service is supported by Google Inc.








