 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > The second most common neurodegenerative disease: Parkinson's disease Parkinson's disease (PD) is the second most common neurodegenerative disease affecting middle-aged and older adults. The primary clinical symptoms include resting tremor, rigidity, bradykinesia, impaired gait, and posture.
The incidence of Parkinson's disease increases with age, and the average PD onset age is estimated to be 60 years old, and only about 4% of PD patients are diagnosed before the age of 50. In addition, men are 1.5 times more likely to develop Parkinson's disease than women. Published reports show that in China, the population over 65, there is a prevalence of Parkinson's disease of approximately 1.7%. Furthermore, the majority of Parkinson's cases develop randomly, with less than 10% having a hereditary link.
According to the data from Frost & Sullivan, in China, the number of people over the age of 65 with PD continues to grow, reaching 2,831,000 in 2018. This number is projected to increase to 3,459,000 in 2023. The latest literature showed that the number of PD cases in the United States is expected to increase to more than 1 million by 2030. Therefore, there is a huge demand for a novel and effective treatment for PD patients worldwide.
The etiology of PD is not fully understood; the risk of developing PD lies on the crosstalk between genetic risk factors and environmental factors. Key pathological hallmarks of PD are degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) and abnormal aggregation of alpha-synuclein (SNCA), which is the major component of Lewy bodies.
SNCA, a 140-amino acid presynaptic protein, is involved in neuronal plasticity, membrane vesicle processing, and neurotransmitter release. SNCA has three distinct domains: an N-terminal lipid-binding domain, a central non-beta-amyloid component, and an acidic C-terminal domain.
Abnormal SNCA is associated with multiple neurological dysfunction and degeneration mechanisms, including inflammation, impaired mitochondrial function, altered protein degradation systems, and oxidative stress. Specifically, normal SNCA is in the form of unfolded monomers. In PD, SNCA undergoes incorrect post-translational modifications such as phosphorylation at the Ser129 site leading it to fold into dimers, trimers, and oligomers, although it is not clear which polymerization form directly induces the neurotoxicity. These polymers further aggregate into protofibrils and amyloid fibrils that accumulate within neurons, impairing neuronal function and finally leading to neuron death. This process causes inflammation, resulting in further tissue damage.
SNCA has emerged as a key target for developing new PD therapies in sporadic and familial PD.
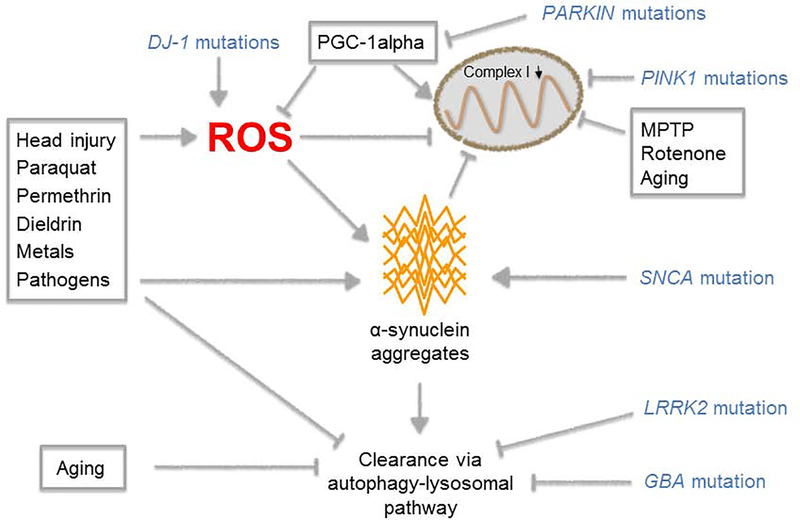
Multiple pathways that influence the onset of PD
Therapies targeting SNCA that aim to reduce SNCA levels directly or indirectly or modulate the subsequent inflammatory process are currently under development. Immunotherapy targeting SNCA can either be passive immunization or active immunization.
Immunotherapy targeting SNCA
Active immunization is a classical vaccination strategy that uses SNCA antigens as immune stimulants to activate a prolonged humoral response and trigger specific antibody production.
Progress in SNCA-targeted vaccine Development
| Drug Name | Status | Indications | Company |
|---|---|---|---|
| ACI-7104 | Phase I | Parkinson's Disease | Ac Immune |
| UB-312 | Phase I | Parkinson's Disease; Parkinsonism | United Neuroscience |
| Affitope-PD01 | Phase I | Multiple Sclerosis; Parkinson's Disease; Neurodegenerative disease | Affiris |
| PV-1950 | Preclinical | Parkinson's Disease | Institute For Molecular Medicine; Nuravax |
| Affitope-PD03 (AFFiRiS) | No advance | Multiple Sclerosis; Parkinson's Disease; Neurodegenerative disease | Affiris |
In passive immunotherapy, several anti-SNCA antibody drugs are currently in Phase II, Phase I, and preclinical stages of clinical trials. The results of Phase I clinical trial of Cinpanemab/BIIB054 proved to be safe and tolerable, but the Phase II clinical trial was terminated due to safety issues that did not adequately meet the primary and secondary focus. Prasinezumab appears to lead to positive results, possibly due to the different binding sites of SNCA, with Cinpanemab binding to the N terminus, while Prasinezumab binds to the C terminus of the SNCA.
Progress in SNCA-targeted biologic drug Development
| Drug Name | Target | Drug/Therapy Type | Status | Indications | Company |
|---|---|---|---|---|---|
| Prasinezumab | SNCA | Humanized monoclonal antibody | Phase II | Parkinson's Disease | Prothena |
| Lu AF-82422 (Lundbeck A/S) | SNCA | Monoclonal antibody | Phase II | Multiple Sclerosis; Parkinson's Disease | Lundbeck; Genmab |
| UCB-7853 | SNCA | Monoclonal antibody | Phase I | Parkinson's Disease | Ucb Biopharma Srl |
| MEDI-1341 | SNCA | Antibody | Phase I | Parkinson's Disease | AstraZeneca plc; Takeda Pharmaceutical Co Ltd |
| Anti-a-syn antibody | SNCA | Antibody | Pre-clinical | Parkinson's Disease | Ac Immune |
| ATV:aSyn | SNCA | Antibody | Pre-clinical | Parkinson's Disease | Denali Therapeutics Inc |
| ABL-301 | IGF1R; SNCA | Bispecific antibody | Pre-clinical | Parkinson's Disease | Abl Bio |
| PR-004 | GlcCer; SNCA | Genetic therapy | Pre-clinical | Neurodegenerative disease | Eli Lilly |
Existing research still does not meet the clinical needs of Parkinson's disease care. Compared to the motor symptoms, the underlying neuropharmacology of symptoms is minimally understood. In addition, the predictive value and optimal application of preclinical models have not been thoroughly studied, and new evaluable drugs are often lacking in clinical studies. It is hoped that scientific drilling and technological innovation will provide better symptomatic and disease-modifying treatment for PD patients.
ACROBiosystems has SNCA/Alpha-Synuclein protein(Met 1 - Ala 140)to support the research and development of PD therapeutic drugs.
| Cat. No. | Species | Product Description |
|---|---|---|
| ALN-H5253 | Human | Human Alpha-Synuclein Protein, Fc Tag (MALS verified) |
| ALN-H52H8 | Human | Human Alpha-Synuclein Protein, His Tag |
| ALN-H82H8 | Human | Biotinylated Human Alpha-Synuclein Protein, His,Avitag™ |
| ALN-C52H5 | Cynomolgus | Cynomolgus Alpha-Synuclein Protein, His Tag (MALS verified) |
SDS-PAGE is used to verify high-purity SNCA/Alpha-Synuclein proteins.
>>>Learn more about AneuRO: proteins for neuroscience
ACRO is developing more proteins for AD diagnosis and treatment. If you have any needs or more development suggestions, don't hesitate to get in touch with us.
1,S.H. Fox, J.M. Brotchie. Special Issue on new therapeutic approaches to Parkinson disease. Neuropharmacology(2022). https://doi.org/10.1016/j.neuropharm.2022.108998
2,Fleming, S.M., Davis, A., Simons, E. Targeting alpha-synuclein via the immune system in Parkinson's disease; current Vaccine therapies Neuropharmacology(2022). https://doi.org/10.1016/j.neuropharm.2021.108870
3,Simon, D. K., Tanner, C. M., & Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clinics in geriatric medicine (2020). https://doi.org/10.1016/j.cger.2019.08.002
4,Galvan, A., Devergnas, A., & Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state (2015). https://doi.org/10.3389/fnana.2015.00005
This web search service is supported by Google Inc.
