 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Increase or Decrease: The Two Therapeutic Strategies via FcRn-Mediated Mechanism Antibodies are components of the humoral immune response, which is part of the adaptive branch of the immune system. Antibodies are also known as immunoglobulins (Ig), and there are five different types (IgA, IgD, IgE, IgG, and IgM) with varying:
- Molecular weights range from ~150 kDa (IgG) to ~900 kDa (IgM).
- Half-life, ranging from 3 days for IgA and IgD to 23 days for IgG (except for IgG3).
- Immune functions include complement binding ability, immune cell binding affinity, and action sites.
The constant region of the antibody heavy chain determines that IgG has a γ heavy chain, IgM has a μ heavy chain, IgA has an α heavy chain, IgD has a δ heavy chain, IgE has an ε heavy chain. The Fab region is flexible and responsible for antigen binding, while the Fc region is responsible for the binding of cellular receptors located on the surface of cells, conferring its effector function.
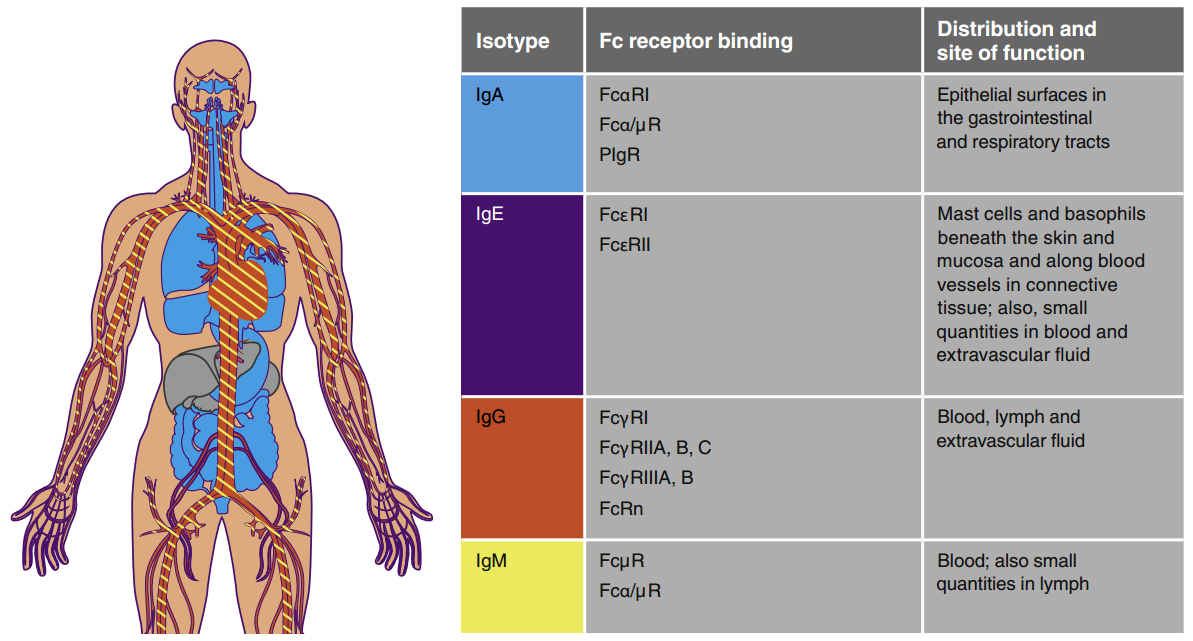
Distribution and function of different immunoglobulins
FcRn is a receptor that binds exclusively to IgG and mediates a recycling mechanism for IgG transport in a pH-dependent manner. (Click here to find out more about the structure of FcRn and its mediated recycling mechanism) Two therapeutic strategies rely on FcRn-mediated mechanisms:
(1)Decrease in the half-life of pathological IgG antibody to treat autoimmune diseases.
(2)Increase in the half-life of therapeutic antibody drugs to extend the therapeutic benefits.
Various autoimmune diseases such as myasthenia gravis (MG), chronic inflammatory demyelinating multiple radiculopathy (CIDP), pemphigus, and primary immune thrombocytopenia are mediated by IgG autoantibodies. For instance, these antibodies play a pathogenic role in MG, leading to the failure of neuromuscular signal transduction by receptors and proteins at neuromuscular junctions. To address this mechanism of autoimmunity, leaders in the field have explored FcRn inhibitors to decrease the pathogenesis of IgG and develop targeted therapies. This approach can potentially decrease endogenous pathogenic IgG concentrations by leveraging the discrepancy in FcRn affinity, resulting in competitive binding with endogenous IgG causing autoimmune disease.
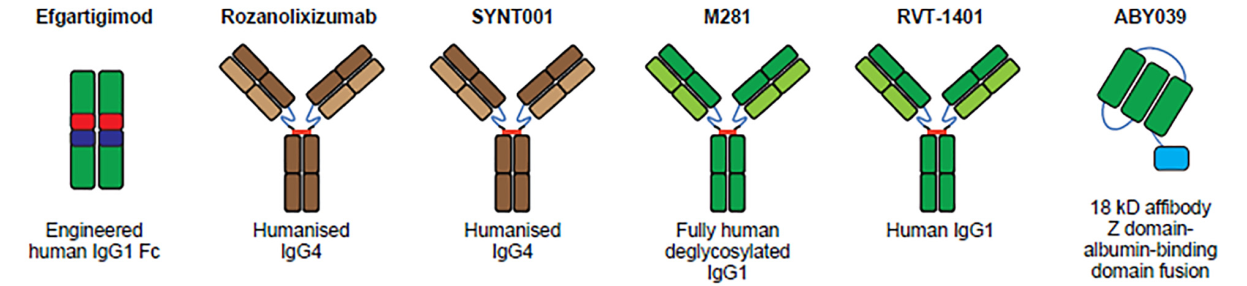
Structure of FcRn targeted drugs
- Efgartigimod is the first and the only FcRn antagonist approved by FDA. Efgartigimod is a modified fragment of human IgG1 Fc that binds to FcRn with high affinity. It is also the first approved therapy specifically targeted at reducing pathogenic IgG, providing a new therapeutic approach for treating gMG. In a Phase I trial, IgG levels were decreased by 50-75% with no effect on albumin levels. This study progressed into a Phase II trial which resulted in 75% of patients showing improvement in a timely manner with minimal to no relapse. Finally, phase III trial studies showed improvement in daily activities in MG patients.
- Rozanolixizuma is a humanized monoclonal antibody derived from UCB Biopharma, a biotechnology company based in Brussels. Rozanolixizuma can specifically bind human FcRn, block the interaction between FcRn and IgG, inhibit the IgG cycle, and induce the clearance of pathogenic IgG autoantibodies. Rozanolixizuma is currently in phase III trials.
- Nipocalimab is an IgG1 anti-FcRn monoclonal antibody developed by Johnson Johnson. This therapeutic decreases IgG levels by up to 90%, showing a slight decrease in total protein and serum albumin levels. However, adverse reactions such as headaches and fever indicated that the tolerability of Nipocalimab needs to be improved.
- Batoclimab (RVT-1401) is a complete human monoclonal antibody derived from Harbour BioMed. Batoclimab binds to FcRn and blocks FcRn-IgG interaction to accelerate the degradation of autoantibodies and treat a variety of pathogenic IgG-mediated autoimmune diseases. This product has been included as a breakthrough therapy in China and is currently undergoing clinical trials for various indications.
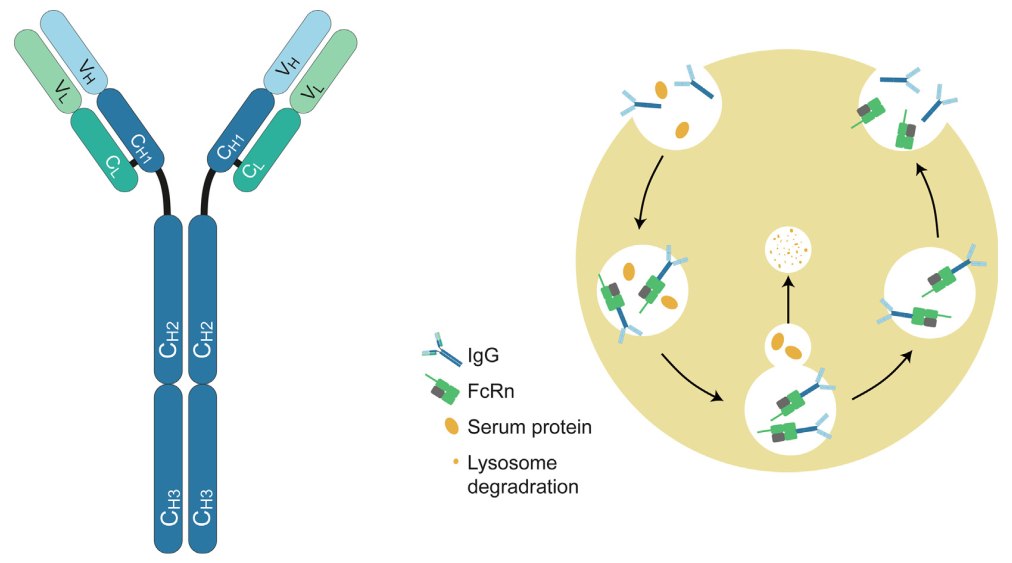
Structure of IgG antibody and FcRn-mediated recycling mechanism
The FcRn pathway has been used to prolong the half-life of therapeutic antibodies. Antibody binding to FcRn can be enhanced using biomolecular engineering technology, fusing Fc fragments, or exchanging amino acids in specific binding bags.
- Etanercept is a treatment consisting of a TNF receptor and an IgG1 Fc fragment approved for rheumatoid arthritis (RA) and other autoimmune diseases. Etanercept can be combined to freely utilize TNFα and β, thereby reducing the levels of these inflammatory factors. During degradation, TNF can be broken down upon release, while Fc fragments can pass through the FcRn pathway, resulting in decreased levels of pro-inflammatory cytokines and a prolonged half-life of therapeutic drugs.
- VRC01 is a neutralizing monoclonal antibody (bnMAb) IgG1 broadly used for HIV-1 prevention. VRC01LS performs two amino acid mutations (M428L and N434S) in the Fc region of VRC01 to extend the serum half-life. LS mutations enhance IgG-FcRn binding, with no effect on FcγRs binding. Hence, VRC01 does not impair Fc-mediated effector functions like antibody-dependent cytotoxicity (ADCC).
- Eculizumab, a monoclonal antibody that inhibits complement protein, C5, is the first drug therapy approved to treat life-threatening thrombotic microangiopathy (TMA) in patients with atypical hemolytic uremic syndrome (aHUS), improving outcomes in patients with aHUS and TMA. However, Eculizumab requires intravenous infusion every 2-3 weeks, increasing the infusion burden and associated adverse events. To overcome this problem, a long-effective anti-C5 monoclonal antibody (Ravulizumab, Ultomiris®) engineered based on Eculizumab offers an important treatment option. Ravulizumab is a combination of Eculizumab with two structural changes designed to extend its terminal elimination half-life. First, two histidine substitutions in the complementary determinate region increased the dissociation rate of the monoclonal antibody C5 complex at pH 6.0, eliminating targeted mediated antibody clearance. Secondly, the affinity of the antibody to human FcRn was enhanced by two amino acid substitutions in the Fc region. Ravulizumab addresses TMA and is well tolerated in adult and pediatric aHUS patients. The mechanism of action is similar to Eculizumab except with a 4-fold increase in action time; hence, minimizing the maintenance dose frequency.
- Satralizumab is a humanized monoclonal antibody targeting IL-6R, and it utilizes a new "antibody recycling technique." Tocilizumab is injected subcutaneously at 162mg per week, while Satralizumab alters the amino acid sequence in the Fc region: mutating a tyrosine residue to a histidine residue, allowing longer antibody cycle time, and thus reducing the frequency of administration, at 120mg per month subcutaneously.(Click here to view previous highlights: 【Inspiring Target】Therapeutic potential of IL-6 immunization in multiple fields continues to burst)
To support affinity validation of antibody drugs and the development of FcRn-targeted antibody drugs for the treatment of autoimmune diseases, ACROBiosystems provides you with a range of high-quality FcRn proteins.
![]() Expressed by HEK293
Cells: to achieve post-translational modification and proper protein folding
Expressed by HEK293
Cells: to achieve post-translational modification and proper protein folding
![]() Variousspecies: Human, Mouse, Cynomolgus/Rhesus macaque, Rat, Porcine, Rabbit, Feline, Bovine, canbe fully applied to different cross-species experiments
Variousspecies: Human, Mouse, Cynomolgus/Rhesus macaque, Rat, Porcine, Rabbit, Feline, Bovine, canbe fully applied to different cross-species experiments
| more than 95% as verified by SDS-PAGE |
more than 90% as verified by SEC-MALS |
![]() Low endotoxin:<1.0
EU/µg
Low endotoxin:<1.0
EU/µg
![]() Biotinylated FcRnproteins labeled with AvitagTMoffered. High labeling efficiency, specificity, and clarity. Suitable for ELISA/SPR/BLIdetection based on binding to streptavidin for drug development and optimization process
Biotinylated FcRnproteins labeled with AvitagTMoffered. High labeling efficiency, specificity, and clarity. Suitable for ELISA/SPR/BLIdetection based on binding to streptavidin for drug development and optimization process
![]() Affinity verified by SPR BLI:bioactivity is tested and guaranteed, and protocols are offered freeof charge.
Affinity verified by SPR BLI:bioactivity is tested and guaranteed, and protocols are offered freeof charge.
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|---|---|---|---|
| FcRn (FCGRT B2M) | FCM-H5286 | HEK293 | Human FcRn / FCGRTB2M Heterodimer Protein, His TagStrep II Tag (SPR MALS verified)Hot |  |
| FCM-H8286 | HEK293 | Biotinylated Human FcRn / FCGRTB2M Heterodimer Protein, His TagStrep II Tag, ultra sensitivity (primary amine labeling) (SPR verified) |  | |
| FCM-H82W4 | HEK293 | Biotinylated Human FcRn / FCGRTB2M Heterodimer Protein,,His TagStrep II Tag (SPR BLI MALS verified)Hot |  | |
| FCM-H82W7 | HEK293 | Biotinylated Human FcRn / FCGRTB2M Heterodimer Protein, His,(MALS SPR verified) |  | |
| FCN-H52W7 | HEK293 | Human FcRn / FCGRTB2M Heterodimer Protein, His Tag (SPR BLI MALS verified)Hot |  | |
| FCM-M52W2 | HEK293 | Mouse FcRn / FCGRTB2M Heterodimer Protein, His TagTwin-Strep Tag (MALS BLI verified) |  | |
| FCM-M52W8 | HEK293 | Mouse FcRn / FCGRTB2M Heterodimer Protein, His Tag (MALS BLI verified) |  | |
| FCM-M82W5 | HEK293 | Biotinylated Mouse FcRn / FCGRTB2M Heterodimer Protein, His,(MALS BLI verified) |  | |
| FCM-M82W6 | HEK293 | Biotinylated Mouse FcRn / FCGRTB2M Heterodimer Protein,,His TagTwin-Strep Tag (MALS BLI-verified) |  | |
| FCM-R5287 | HEK293 | Rat FcRn / FCGRTB2M Heterodimer Protein, His TagStrep II Tag (MALS SPR verified) |  | |
| FCM-R82W7 | HEK293 | Biotinylated Rat FcRn / FCGRTB2M Heterodimer Protein,,His TagStrep II Tag (MALS SPR verified) |  | |
| FCM-R52W5 | HEK293 | Rabbit FcRn / FCGRTB2M Heterodimer Protein, His TagTag Free (MALS SPR verified) |  | |
| FCM-C5284 | HEK293 | Cynomolgus / Rhesus macaque FcRn / FCGRTB2M Heterodimer Protein, His TagStrep II Tag (MALS BLI verified) |  | |
| FCM-C52W9 | HEK293 | Cynomolgus / Rhesus macaque FcRn / FCGRTB2M Heterodimer Protein, His Tag (MALS BLI verified) |  | |
| FCM-C82W3 | HEK293 | Biotinylated Cynomolgus / Rhesus macaque FcRn / FCGRTB2M Heterodimer Protein, His,(MALS SPR verified) |  | |
| FCM-C82W5 | HEK293 | Biotinylated Cynomolgus / Rhesus macaque FcRn Heterodimer Protein, His,Strep II Tag (SPR BLI verified) |  | |
| FCM-P5280 | HEK293 | Porcine FcRn / FCGRTB2M Heterodimer Protein, His TagStrep II Tag (MALS SPR verified) |  | |
| FCN-F82W3 | HEK293 | Biotinylated Feline FcRn / FCGRTB2M Heterodimer Protein, His,(MALS SPR verified) |  | |
| FCN-B82W3 | HEK293 | Biotinylated Bovine FcRn / FCGRTB2M Heterodimer Protein, His,(MALS SPR verified) |  |
>>>
Click here to find out more high-quality FcRn proteins and verification data with free protocols
Parallel to the previous work, ACROBiosystems has also been developing a series of IgG Fc proteins, containing only the hinge region, CH2 and CH3 sequences, without the Fab sequences. These IgG Fc proteins can be used as ideal isotype controls for monoclonal antibodies, IgG-like bispecific antibodies, ADC, and IgG Fc- fusion drugs. Applications of this product include drug screening, functional verification, etc. In addition, our IgG Fc proteins are used as endogenous IgG for competitive validation of FcRn binding to FcRn targeted antibody drugs.
![]() Expressed by HEK293
cells: for post-translational glycosylation, modifications, and correct protein folding
Expressed by HEK293
cells: for post-translational glycosylation, modifications, and correct protein folding
Human IgG1 Fc,IgG2 Fc,IgG3 Fc,IgG4 Fc | |
Mouse IgG1 Fc,IgG2a Fc,IgG2b Fc | |
Llama IgG2b Fc,Rabbit IgG Fc |
![]() Various tags: Tag Free,AvitagTM,His Tag,gDTag,Flag Tag,AvitagTM His tag
Various tags: Tag Free,AvitagTM,His Tag,gDTag,Flag Tag,AvitagTM His tag
![]() Low endotoxin: Less
than 1.0 EU/μg by the LAL method
Low endotoxin: Less
than 1.0 EU/μg by the LAL method
more than 95% as verified by SDS-PAGE | |
more than 90% as verified by SEC-MALS |
![]() Highbioactivity: verified by ELISA SPR with free protocols
Highbioactivity: verified by ELISA SPR with free protocols
| Molecule | Cat. No. | Species | Product description | Preorder/Order |
|---|---|---|---|---|
| IgG1 Fc | FCC-H5214 | Human | Human IgG1 Fc Protein, Tag Free (MALS verified) | |
| IgG1 Fc | IG1-H5225 | Human | Human IgG1 Fc Protein, His Tag (MALS verified) | |
| IgG1 Fc | IG1-H52C9 | Human | Human IgG1 Fc (C103S) Protein, Flag Tag (MALS verified) | |
| IgG1 Fc | IG1-H52G6 | Human | Human IgG1 Fc (C103S) Protein, gD Tag (MALS verified) | |
| IgG1 Fc | IG1-H8213 | Human | Biotinylated Human IgG1 Fc protein,(MALS verified) | |
| IgG1 Fc | IG1-H82E9 | Human | Biotinylated Human IgG1 Fc (C103S) Protein,,His Tag | |
| IgG1 Fc | IG1-M5208 | Mouse | Mouse IgG1 Fc Protein, Tag Free (MALS verified) | |
| IgG1 Fc | IG1-M8211 | Mouse | Biotinylated Mouse IgG1 Fc protein,(MALS verified) | |
| IgG2 Fc | IG2-H5206 | Human | Human IgG2 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG2a Fc | IGA-M5207 | Mouse | Mouse IgG2a Fc Protein, Tag Free (MALS verified) | |
| IgG2a Fc | IGA-M8210 | Mouse | Biotinylated Mouse IgG2a Fc Protein,(MALS verified) | |
| IgG2b Fc | IGB-M5203 | Mouse | Mouse IgG2b Fc Protein, Tag Free (MALS verified) | |
| IgG2b Fc | IGB-L5204 | Llama | Llama IgG2b Fc Protein, Tag Free (MALS verified) | |
| IgG3 Fc | IG3-H5200 | Human | Human IgG3 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG3 Fc | IG3-M5214 | Mouse | Mouse IgG3 Fc Protein, Tag Free (MALS verified) | |
| IgG4 Fc | IG4-H5205 | Human | Human IgG4 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG Fc | IGG-R5203 | Rabbit | Rabbit IgG Fc Protein, Tag Free (MALS verified) |
>>> Click here to find out more IgG Fc proteins and verification data with free
protocols
1. Hans-Hartmut Peter, Hans D. Ochs, Charlotte Cunningham-Rundles, et al. Targeting FcRn for immunomodulation: Benefits, risks, and practical considerations. Journal of Allergy and Clinical Immunology. 2020, 146(3): 479-491. https://doi.org/10.1016/j.jaci.2020.07.016.
2. Adrian W. Zuercher, Rolf Spirig, Adriana Baz Morelli et al. Next-generation Fc receptor–targeting biologics for autoimmune diseases, Autoimmunity Reviews, 2019,18(10). https://doi.org/10.1016/j.autrev.2019.102366
3. Yahiya Y. Syed. Ravulizumab: A Review in Atypical Haemolytic Uraemic SyndromeDrugs. 2021, 81: 587–594. https://doi.org/10.1007/s40265-021-01481-6
4. Gil I. Wolfe, E. Sally Ward, Hans de Haard et al. IgG regulation through FcRn blocking: A novel mechanism for the treatment of myasthenia gravis, Journal of the Neurological Sciences, 2021, 430. https://doi.org/10.1016/j.jns.2021.118074
5. Johann Sellner, Harald H. Sitte, Paulus S. Rommer. Targeting interleukin-6 to treat neuromyelitis optica spectrum disorders: Implications from immunology, the FcRn pathway and clinical experience, Drug Discovery Today, 2021, 26(7):1591-1601, https://doi.org/10.1016/j.drudis.2021.03.018
This web search service is supported by Google Inc.
