Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > SARS-CoV-2 variants, immune escape and multivalent vaccine candidates: What ACRO offers to solve the biggest problem The emergence and persistence of SARS-CoV-2 variants pose a continuing threat to global health. Since the first potent spike mutation, D614G, was identified in February 2020, a significant number of spike mutations that alter virus fitness were successively characterized. To better track and report the evolution of the virus, the WHO listed four dominantly circulating variants as “Variants of Concerns (VOCs)”, namely Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2). These variants carry “evolutionarily important” mutations on spike that confer higher viral load, faster transmission and stronger resistance to established immunity.
As cases of infection with SARS-CoV-2 variants rapidly increases and take over the trend of the pandemic, a concerning question arises: can marketed vaccines, developed based on the wild-type viral strain, still provide enough protection against SARS-CoV-2 variants?
The answer may not be positive. Recently, variants were reported as the important causes of breakthrough infections. According to the latest study published by the Centers for Disease Control and Prevention (CDC) on August 27th, 2021, the Delta variant gradually became the dominant variant from December 2020 to August 2021. During this period, the vaccine effectiveness in fully vaccinated medical staff has decreased from 91% to 66%.
Therefore, it is urgent to develop vaccines that protect against SARS-CoV-2 variants in response to the risks. On January 26, 2021, Moderna announced the development of their second COVID-19 mRNA vaccine, mRNA-1273.351, which effectively protects against the Beta variant as a strain-matched booster. In May 2021, data from phase 2 clinical study of this vaccine has been submitted to FDA and EMA for approval. Other vaccine developers are also attempting to develop multivalent vaccine candidate. China National Biotec Group (CNBG) has just completed preclinical studies of a COVID-19 vaccine against the Beta and Delta variants and is applying for NMPA clinical trial approval.
In June 2020, the U.S. Food and Drug Administration (FDA) released a document entitled “Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry” to address the standards set forth for COVID-19 preventive vaccine development and approval. The guidelines state that the establishment of a method for quantitative analysis of SARS-CoV-2 antigen content is necessary in the process of vaccine efficacy evaluation and quality control.
Click to download the FDA guidance for development and licensure of COVID-19 vaccines >>>
To support the global COVID-19 response and vaccine development, ACROBiosystems developed a broad-spectrum SARS-CoV-2 antigen detection kit based on sandwich ELISA method, which is designed as a highly efficient tool for the quality control stage of COVID-19 vaccine production to meet the needs of vaccine companies for quantitative detection of SARS-CoV-2 antigen content.
Customized sensitivity to meet different detection needs at customized sensitivity
Can recognize mutant antigens from multiple strains at equal sensitivity
Provide kits for different epitopes detection (Spike trimer and Spike RBD)
High batch-to-batch stability ensured consistency of detection in the mass manufacturing and quality control process
It has been verified that ACRO's antigen detection kits can broadly identify Spike Protein and RBD antigens of both wild-type and different varints with similar binding activity in ELISA.
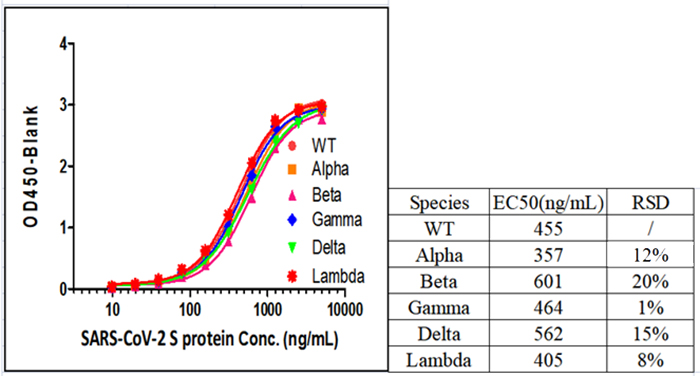
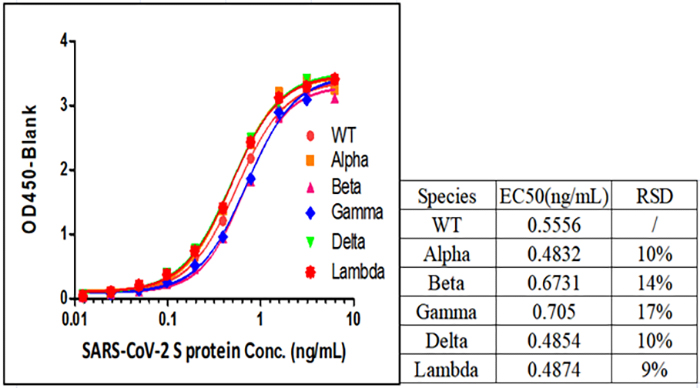
Fig 1. SARS-CoV-2 Spike Protein ELISA Kit (For Vaccine Development) (Cat.No.RAS-A039) can detect all SARS-CoV-2 Variants of Concerns (VOCs), including Alpha (Cat.No.SPN-C52H6), Beta (Cat.No.SPN-C52Hk), Gamma (Cat.No.SPN-C52Hg) and Delta (Cat.No.SPN-C52He).
The kits meet the international standards for the following parameters: Linear curve range, Reportable range, Precision, Accuracy
results (OD450 nm-OD630 nm) were analyzed to evaluate the precision of the method. Deviation between each test of the quality control samples should be ≤15%.
| Cat. No. | High Conc. Quality Control | Medium Conc. Quality Control | Low Conc. Quality Control | Results |
|---|---|---|---|---|
1.0% | 2.0% | 1.7% | Meet the standards (CV≤15%) | |
1.4% | 2.1% | 3.1% | Meet the standards (CV≤15%) |
table 1. Evaluation of Intra-assay precision for RAS-A021 and RAS-A039
| Cat. No. | High Conc. Quality Control | Medium Conc. Quality Control | Low Conc. Quality Control | Results |
|---|---|---|---|---|
2.9% | 3.1% | 3.2% | Meet the standards | |
1.2% | 1.0% | 1.0% | Meet the standards |
table 2. Evaluation of Inter-assay precision for RAS-A021 and RAS-A039
With confounding factors strictly under control (same operator, same laboratory, same procedures), 10 accuracy tests were performed to analyze the same quality control samples of high, medium, and low concentration. The test results (OD450 nm-OD630 nm) were analyzed to evaluate the accuracy of the method. Deviation between the calculated value and the theoretical value of the quality control samples should be ≤15%.
| Cat. No. | High Conc. Quality Control (ng/mL) | High Conc. Quality Control Result (AVE) | Accuracy | Medium Conc. Quality Control (ng/mL) | Medium Conc. Quality Control Result (AVE) | Accuracy | Low Conc. Quality Control (ng/mL) | Low Conc. Quality Control Result (AVE) | Accuracy | Results |
|---|---|---|---|---|---|---|---|---|---|---|
0.517 | 0.5 | 3% | 0.28 | 0.25 | 12% | 0.138 | 0.125 | 11% | Meet the standards (CV≤15%) | |
484 | 500 | -3% | 259 | 350 | 4% | 126 | 125 | 1% | Meet the standards (CV≤15%) |
table 3. Evaluation of accuracy for RAS-A021 and RAS-A039
This web search service is supported by Google Inc.