 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Critical importance for the evaluation of the Fc domain binding activity Special Fc function domain
Antibody drugs has quickly become the focus of global drug research and development due to high specificity and potency against intended targets. Antibody drugs are based on engineered immunoglobulin which are composed of the fragments antigen binding (Fab) domain, mainly responsible for recognizing and binding antigens, and the fragment crystallizable (Fc)-fusion domain that binds to effector molecules or interacts with cells to exert toxic effects. Once Fab domain has been successfully engineered against a particular antigen, the focus of antibody drug development and research centers mainly on Fc domain optimization.
Fc crystallizable (Fc)-fusion molecules are often engineered with a specific immunoglobulin G (IgG) isotype and subtype in order to impart particular functionalities. These include half-life extension and induction of cytotoxic activity[1]. Alternatively, a specific isotype and subtype may be selected to mitigate, or lessen, potential induction of unwanted biological activities. These modalities consist of specific structural/functional domains that result in the intended effect and improve therapeutic benefit.
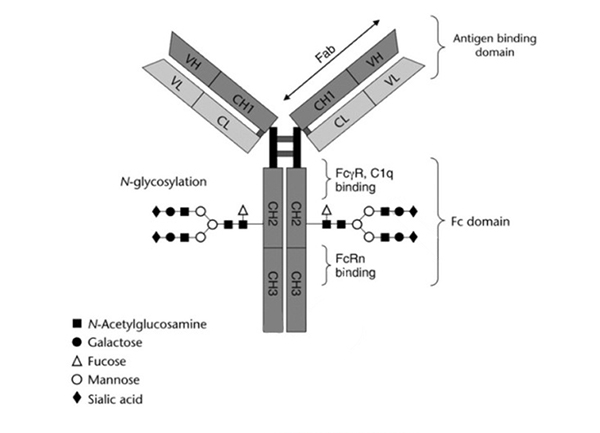
Figure 1. Basic structure of an IgG1 antibody. All the function domain available for engineering is indicated including antigen binding via the fragment antigen binding (Fab), Fc gamma receptor (FcγR) binding, complement component 1q (C1q) binding, and neonatal Fc receptor (FcRn) binding domains.[2]
Fc-mediated effector function activities
The efficacy of a therapeutic antibody not only depends on the activity of the Fab fragments, but also on the interaction of the Fc fragments with cellular Fc receptors. The Fc fragment of the antibody affects the pharmacokinetics and drug toxicity in-vivo by binding to Fc receptors and Clq complex. The functional effects mediated by Fc mainly include the following:
1. Antibody-dependent cell-mediated cytotoxicity (ADCC)
ADCC is a cell-mediated innate immune mechanism whereby an effector cell of the immune system (e.g., Natural Killer (NK) cells, monocytes, macrophages, or eosinophils) bind to antibody/target cell complex inducing cell lysis through release of cytotoxic factors as shown in Fig. 2. The NK cells contain FcγR and most commonly bind the antibody Fc region with FcγRIII/CD16 receptors.
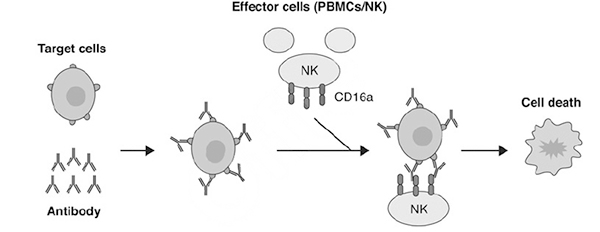
Figure 2. Process of ADCC begins with binding of antibodies to the intended target on a cell surface followed by immune effector cells like NK cell or other peripheral blood mononuclear cells (PBMCs) binding to the complex and initiating cell lysis and death.[2]
2. Complement-dependent cytotoxicity (CDC)
CDC represents another mechanism by which a therapeutic monoclonal antibody can destroy target cells depending on the complex formation with C1q resulting in a membrane attack complex which induced target cell lysis (Fig. 3).
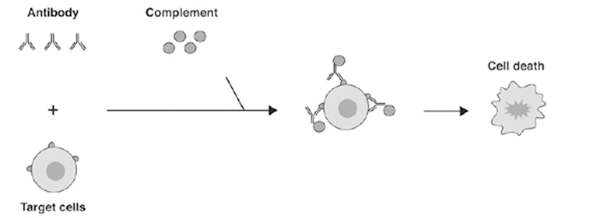
Figure 3. CDC begins first with antibody binding to target antigen expressing cells followed by C1q binding, MAC formation and cell lysis.[2]
3. Antibody-dependent cellular phagocytosis (ADCP)
ADCP is an Fc-dependent, cell-mediated, and innate immunity mechanism in which antibody-opsonized target cells, or in some cases, specific fibrillar proteins, engage the FcγR on the surface of immune effector cells like macrophages or monocytes to induce phagocytosis as shown above (Fig. 4).
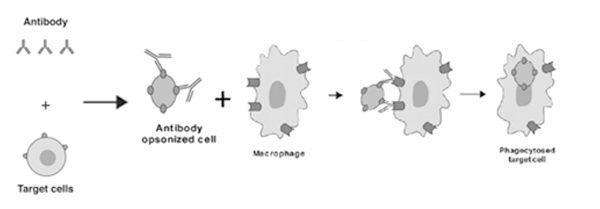
Figure 4. ADCP results from macrophage binding of antibody/target cell complex resulting in phagocytosis and cell lysis.[2]
4. Cross linking antibody-dependent apoptosis
Cross linking secondary antibody-dependent apoptosis is another Fc effector function in which binding of target cell surface antigen by antibodies in the presence of cross-linking secondary antibodies or FcγR-expressing cells initiates signal transduction events that lead to apoptosis of the cells (Fig. 5).
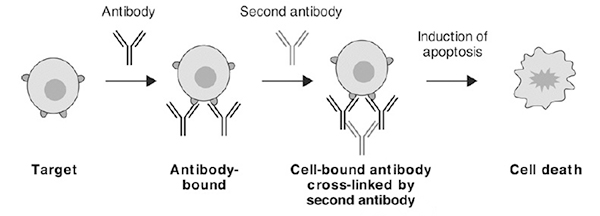
Figure 5. Cross-linking antibody dependent apoptosis occurs after primary antibody bound to target antigen containing cells are polymerized by a secondary antibody binding to the Fc domain leading to cell death.[2]
Non-cell-based binding assay Formats for evaluating the function of the Fc region
Since the binding of Fc receptors can affect Fc-mediated functional effects, engineering of the Fc domain is very important for the characterization of the activity of Fc containing antibody drugs to evaluate its activity, effectiveness and safety. The main role of Fc-containing therapeutic antibody drugs is to engage the FcγR subclass. Since FcγRs contain many different subtypes binding to specific subtypes of Fc domains, the Fc-FcγR interaction is highly complex and in-vitro detection of Fc-Fc receptor binding can predict the functional potency of these antibody drugs. According to the United States Pharmacopoeia, enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR) and biological layer interference (BLI) are all valid assays to assess Fc-Fc receptor binding.
Enzyme-linked immunosorbent assay (ELISA) is an immunoassay technology developed by combining the specificity of the antigen-antibody reaction with the high-efficiency catalysis of detection enzymes. It can be used to detect macromolecular antigens and specific antibodies.
Surface Plasmon Resonance (SPR) technology is a biosensing analytical technology based on the physical optical phenomenon of surface plasmon resonance, centered on biosensing gold plated chips, and is also included in the Chinese, American and Japanese Pharmacopoeias.
Bio-Layer Interferometry (BLI) technology is an optical analysis method (optical interferometry) that relies on fiber optic biosensors to measure changes in light wavelength interference patterns based on binding events.
Table 1. Introduction of ELISA, SPR and BLI
Assay Format | Merits | Limitations | Points to Consider |
SPR | Label-free formats; precise kinetic data; can measure a wide range of binding affinities; automated data analysis possible. | Medium throughput; requires specialized equipment and technical expertise; harder to transfer. | Need high quality of critical reagents such as FcγR, FcRn, and Fc-containing reagents; types of biosensor chip and alternate approaches to immobilization of ligand; minimize steric hindrance and non-specific interactions; avoid avidity artifacts due to overloading ligand on the chip; flowing a higher concentration of analyte over the chip surface to mitigate low-affinity interactions (e.g., FcγRII, FcγRIII). |
BLI | Label-free format; high throughput; can provide kinetic and quantitation data; semi-automatable. | More difficult to transfer; less sensitive than SPR. | Selection of the appropriate biosensor and assay orientation;loading of ligand molecules on the biosensors; selection of buffer conditions to promote optimal and reduce non-specific binding; optimize length of the association step for secondary interaction consideration. |
ELISA | High throughput, automatable; economical, easily transferred. | Multiple wash steps; requires plate precoating; binding limitations. | Presence of aggregates may complicate the interpretation of results; when the binding interactions are weak (e.g., FcγRII and FcγRIII), consider cross-linking to improve sensitivity; critical reagent quality and concentration such as FcγR and FcRn. |
Fast and reliable services---supporting full-cycle of drug development
ACROBiosystems is a global brand focusing on protein technology, products and services in the development of biological drugs. ACRO is committed to providing target antigens and other key reagents and related services required in the development of targeted therapeutic drugs.
Relying on a professional technical team and validated Biacore and ForteBio Octet platforms, ACRO is committed to providing our customers with high-quality integrated molecular interaction analysis and testing services, including antibody (mAb/BsAb/ADC) screening, characterization, and consistency evaluation, Fc receptor affinity detection and analysis, and biomolecules interactions and small molecule affinity testing, etc., to accelerate development of powerful antibody drugs.
Our advantages:
1. Comprehensive protein pipeline support⸺Thousands of high-quality antigens available for experimental selection
2. Experienced R&D technical team - Multiple proven methods to choose
3. Efficient project operations team ⸺Next day reports at the earliest
4. Personalized solution design and report format ⸺Data quality meets filing requirements, supports the Sino-US dual reporting
5. Quality Certification⸺ High standard quality management control system
6. Multi-testing platforms--Multi-platforms can provide testing services
ACROBiosystems Testing and Analysis Service Center launches long-term preferential activities
Provide samples and receive results within two weeks.
Affinity testing experiments covering all genotypes of human Fc receptor proteins required for antibody drug development (as shown below)
All Fc receptor proteins needed for binding experiments available free of charge!
Click here to view Fc receptor proteins 
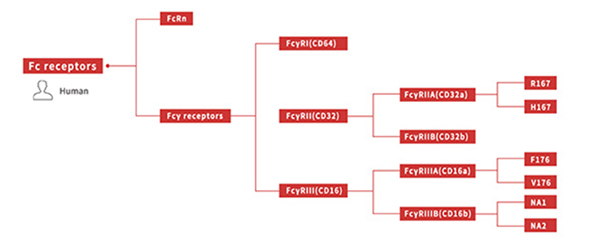
Figure 6. All genotypes of human Fc receptor protein
Case Study
SPR based affinity analysis based on non-mutated IgG1 and human FcγR molecules of different genotypes
Antibody | FcγRs | Cat No. | Species | Analysis method | KD |
Rituximab | Fc gamma RIIB / CD32b | CDB-H5228 | Human | His Capture | 10.1 μM |
Rituximab | Fc gamma RIIA / CD32a | CDA-H5221 | Human | His Capture | 3.12 μM |
Rituximab | Fc gamma RIIA / CD32a | CD1-H5223 | Human | His Capture | 1.45 μM |
Herceptin | Fc gamma RIIIA / CD16a | CD8-H52H4 | Human | His Capture | 163 nM |
Rituximab | Fc gamma RIIIB / CD16b (NA2) | CDB-H5222 | Human | His Capture | 2.88 μM |
Rituximab | Fc gamma RIIIA / CD16a | CDA-H5220 | Human | His Capture | 0.63 μM |
Rituximab | Fc gamma RIIIB / CD16b (NA1) | CDB-H5227 | Human | His Capture | 8.17 μM |
References
[1] Noubia Abdiche Y, et al. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. MAbs 2015;7(2):331–343.
[2] USP<1108>Assay to evaluate fragment crystallizable (Fc)-mediated effector function.
This web search service is supported by Google Inc.
