
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Latest News: FDA approves Pfizer vaccine for emergency use in US on December 11. It was such a significant milestone to get authorization of a vaccine within a year of scientists identifying the new coronavirus. ACROBiosystems proudly supplied all functional COVID-19 antigens for IgG assay in their phase 1/2 study
>>>Download Pfizer's slides on VRBPAC
![]()
Until now, there are about 13 million people worldwide diagnosed with SARS-CoV-2 infected, and the pandemic is still developing. The vaccine against COVID-19 might be the most effective weapon to defeat this disease. According to the World Health Organization, there are 17 vaccines globally in the clinical stage.
On July 1, 2020, Pfizer and BioNTech announced the positive Phase 1/2 results of their jointly developed mRNA vaccine against COVID-19. These preliminary data provide an initial signal that BNT162b1 targeting the RBD SARS-CoV-2 can produce neutralizing antibody responses in humans at or above the levels observed in convalescent sera, and it does so at relatively low dose levels. You can find more details on the preprint website medRxiv. [1]
This mRNA vaccine, BNT162b1, encodes the receptor binding domain (RBD) of the SARS-CoV-2 spike protein, a key target of virus neutralizing antibodies. Their scientist added a Foldon trimerization motif to the end of the RBD sequence to ensure the trimeric structure, which will further increase the immunogenicity effect. A further modification was added to the mRNA sequence to increase the efficiency of in vivo translation (Figure 1).
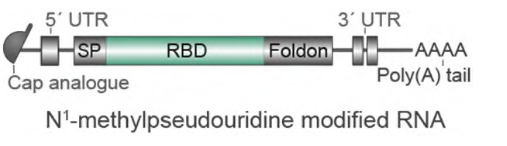
Figure 1. Molecule design of BNT162b1
This phase 1/2 clinical trial was conducted in the United States and Europe at the same time. 45 participants aged between 18 and 55 were randomized and vaccinated. 12 participants per dose level (10ug and 30ug) were vaccinated on Day 1 and 21, and 12 participants received a 100 μg dose on Day 1. 9 participants received a placebo.
Robust immunogenicity was observed after vaccination with BNT162b1. RBD-binding IgG concentrations were detected at 21 days after the first dose and substantially increased 7 days after the second dose given at Day 21. After the first dose, the RBD-binding IgG GMCs (10 µg dose recipients) were similar to those observed in a panel of 38 convalescent, human serology samples obtained at least 14 days after PCR-confirmed following SARS-CoV-2 infection/COVID-19 asymptomatic donors. Dose 2 with 10 µg or 30 µg BNT162b1, the RBD-binding IgG GMCs were about 8-fold to 50-fold that of the convalescent serum panel geometric mean concentrations (GMC).
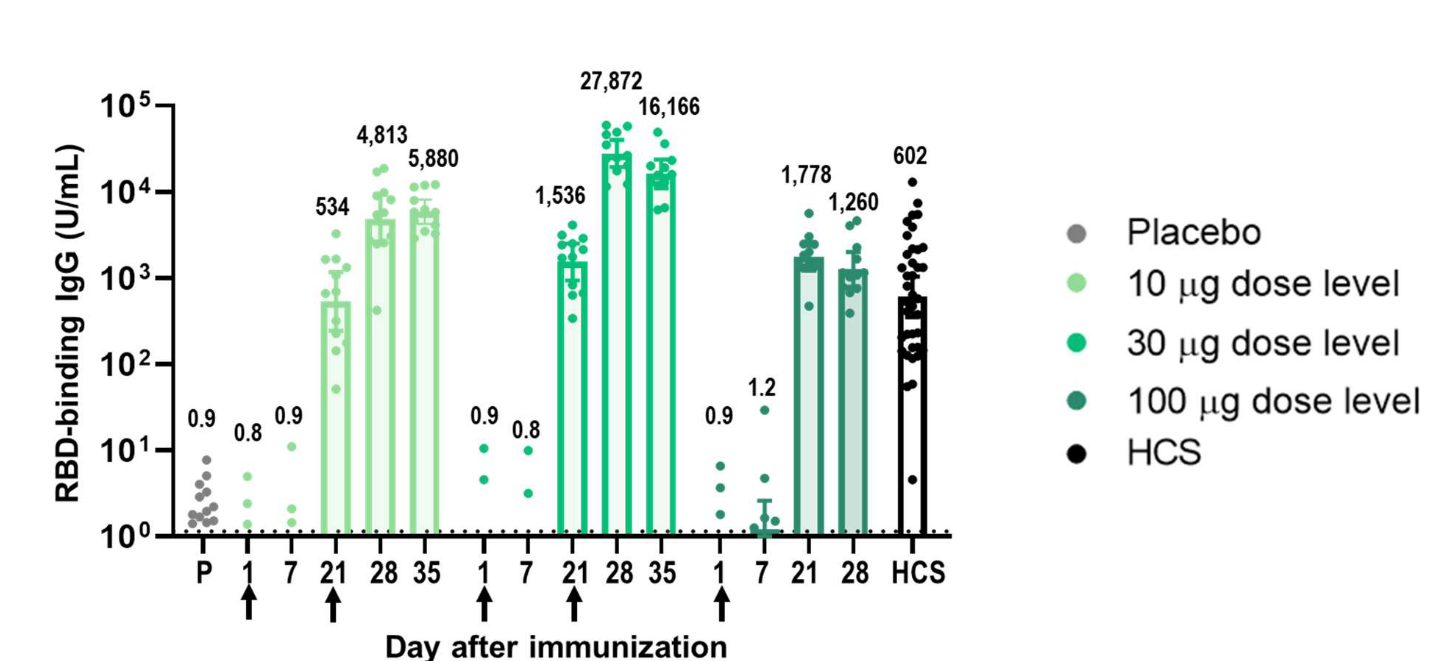
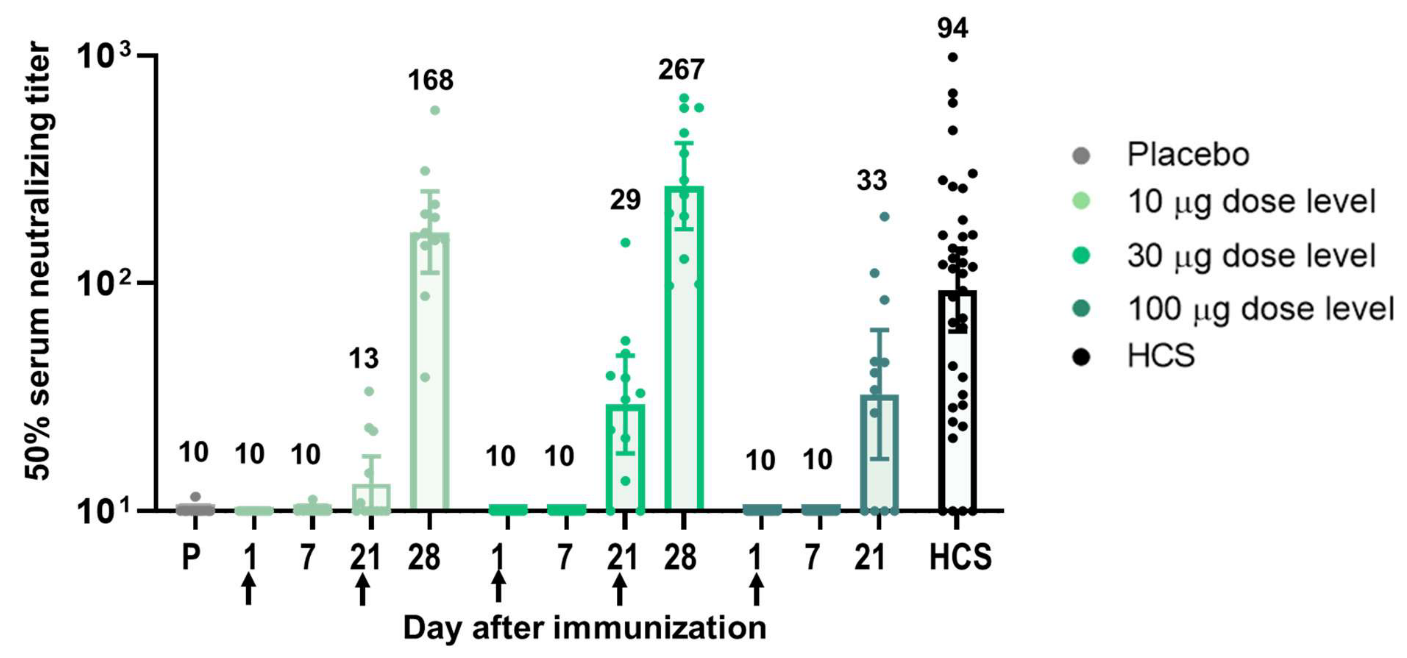
Figure 2. Immunogenicity of BNT162b1
Adverse effects include pain at the injection site, fatigue, headache, chills, muscle pain, joint pain, and fever. Most local reactions and systemic events peaked by the day after vaccination and resolved by Day 7.
This study had several limitations. While they used convalescent sera as a comparator, the kind of immunity (T cells versus B cells or both) and the level of immunity needed to protect from COVID19 are unknown. Further, this analysis of available data did not assess immune responses or safety beyond 2 weeks after the second dose of vaccine. Both are important to inform the public health use of this vaccine. Follow-up will continue for all participants and will include the collection of serious adverse events for 6 months, and COVID-19 infection, and multiple additional immunogenicity measurements through up to two years.
If the ongoing studies are successful and the vaccine candidate receives regulatory approval, the companies expect to manufacture up to 100 million doses by the end of 2020 and potentially more than 1.2 billion doses by the end of 2021. In that event, BioNTech and Pfizer would work jointly to distribute the potential COVID-19 vaccine worldwide.
In the RBD-binding IgG assay, the biotinylated S protein RBD from ACROBiosystems (Cat.No. SPD-C82E9)was bound to streptavidin-coated Luminex microspheres.
ACROBiosystems has developed a series of critical reagents for COVID-19 vaccine development and evaluation, including trimeric S protein which performed a high ratio of signal to noise (S/N), antibodies for reference and CE certified ELISA kits which can make your experiment easier. These featured products are suitable for IgG/M antibody titer detection, neutralizing antibody titer detection and antigen titer detection to accelerate the vaccine development.
>>>Find out more reagents for vaccine development and evaluation
Assay data
※ SDS-PAGE & SEC-MALS: High purity & homogeneity
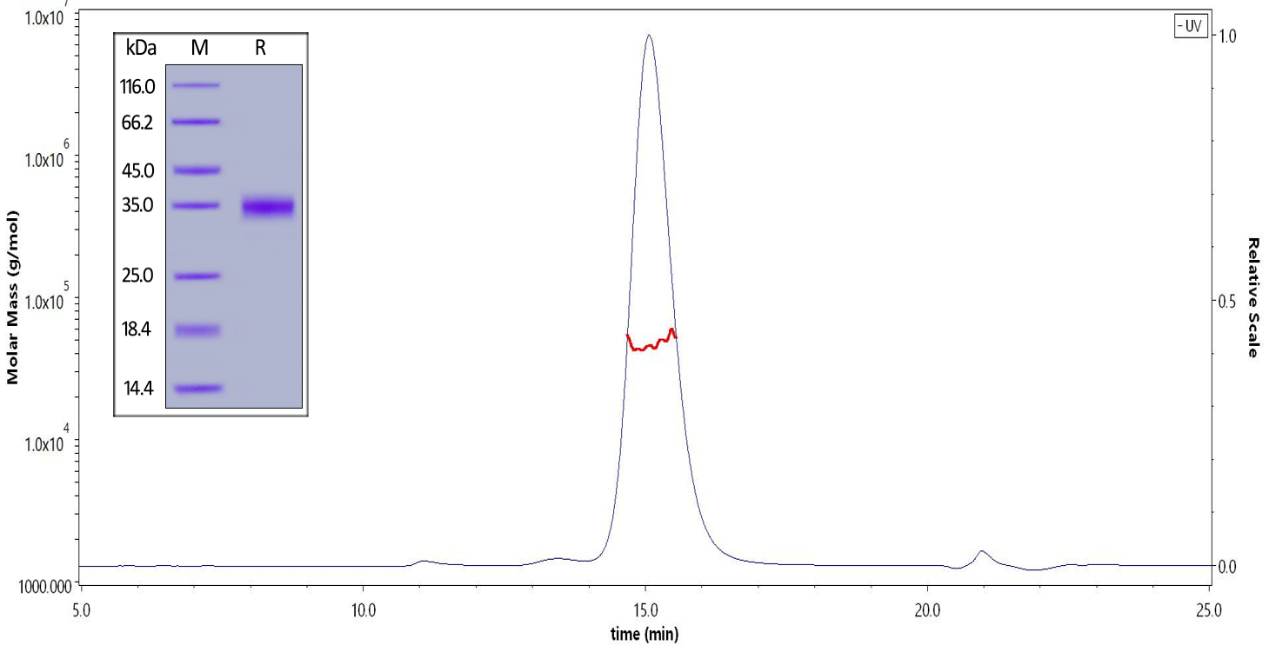
Fig 3 The purity of Biotinylated SARS-CoV-2 S protein RBD, His,Avitag (Cat. No. SPD-C82E9) is greater than 90% verified by SDS-PAGE and SEC-MALS.
※ ELISA: High bioactivity
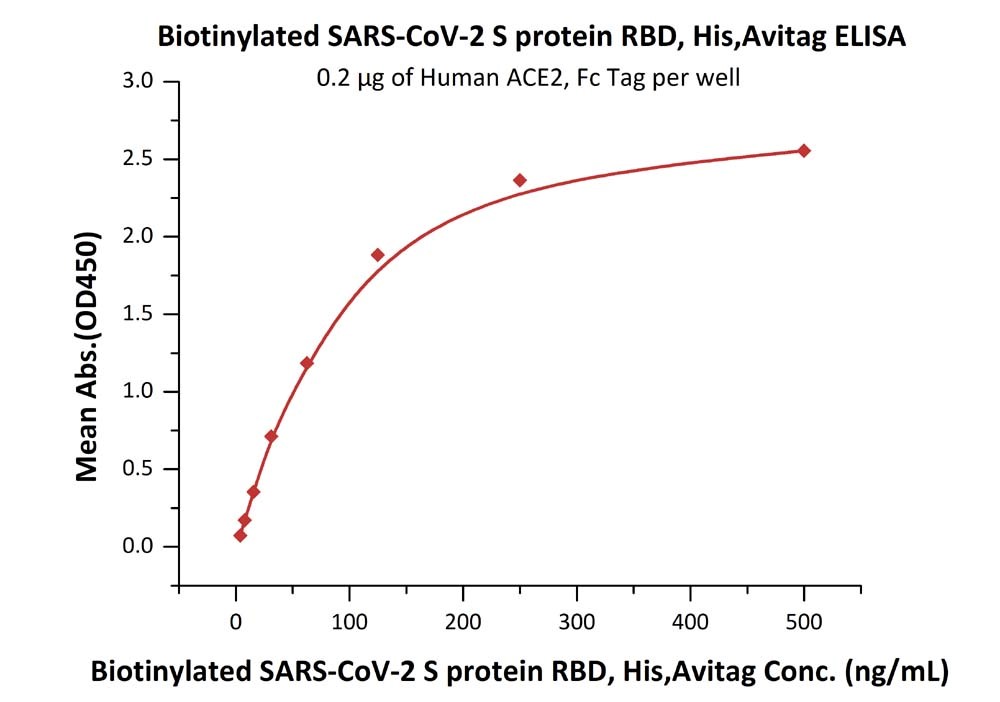
Fig 4 Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 2 μg/mL (100 μL/well) can bind Biotinylated SARS-CoV-2 S protein RBD, His,Avitag (Cat. No. SPD-C82E9) with a linear range of 4-125 ng/mL.
>>Check more SARS-CoV-2 related products
This web search service is supported by Google Inc.







